 | Check left menu for Updates and Revisions!! |
Download Workers’ Compensation Health Care Practice Guidelines:
PART A – Carpal Tunnel
PART B – Chronic Pain
PART C – Cumulative Trauma Disorder
PART D – Low Back
PART E – Shoulder
PART F – Cervical
PART G – Lower Extremities
TITLE 19 LABOR
DELAWARE ADMINISTRATIVE CODE
1000 DEPARTMENT OF LABOR
1300 Division of Industrial Affairs
1340 The Office of Workers’ Compensation
1342 Health Care Practice Guidelines
Table of Contents
PART A CARPAL TUNNEL SYNDROME GUIDELINES - Revised 9/11/2013
1.0 Introduction
2.0 General Guideline Principles
3.0 Definition
4.0 Initial Diagnostic Procedures
5.0 Follow-Up Diagnostic Testing Procedures
6.0 Therapeutic Procedures – Non-Operative
7.0 Therapeutic Procedures - Operative
PART B CHRONIC PAIN TREATMENT GUIDELINES - Revised 9/11/2013
1.0 Introduction
2.0 General Guideline Principles
3.0 Introduction to Chronic Pain
4.0 Definitions
5.0 Initial Evaluation & Diagnostic Procedures
6.0 Therapeutic Procedures – Non-Operative
7.0 Therapeutic Procedures - Operative
8.0 Maintenance Management
PART C CUMULATIVE TRAUMA DISORDER MEDICAL TREATMENT GUIDELINES - Revised 9/11/2013
1.0 Introduction
2.0 General Guideline Principles
3.0 Definitions and Mechanisms of Injury
4.0 Initial Diagnostic Procedures
5.0 Follow-up Diagnostic Imaging and Testing Procedures
6.0 Therapeutic Procedures – Non-Operative
7.0 Operative Treatment
PART D LOW BACK TREATMENT GUIDELINES - Revised 9/11/2013
1.0 Introduction
2.0 General Guideline Principles
3.0 Initial Diagnostic Procedures
4.0 Follow-up Diagnostic Imaging and Testing Procedures
5.0 Therapeutic Procedures - Non-Operative
6.0 Therapeutic Procedures - Operative
7.0 General Guidelines
PART E SHOULDER TREATMENT GUIDELINES - Revised 9/11/2013
1.0 Introduction
2.0 General Guideline Principles
3.0 Introduction to Shoulder Injury
4.0 History Taking and Physical Examination (Hx & PE)
5.0 Specific Diagnosis, Testing and Treatment Procedures
6.0 Therapeutic Procedures - Non-Operative
PART F CERVICAL TREATMENT GUIDELINES - Revised 9/11/20139
1.0 Introduction
2.0 General Guideline Principles
3.0 Initial Diagnostic Procedures
4.0 Diagnostic Imaging and Testing Procedures
5.0 Therapeutic Procedures - Non-Operative
6.0 Therapeutic Procedures - Operative
PART G LOWER EXTREMITY TREATMENT GUIDELINES - Revised 9/11/2013
1.0 Introduction
2.0 General Guideline Principles
3.0 Initial Diagnostic Procedures
4.0 Follow-up Diagnostic Imaging and Testing Procedures
5.0 Specific Lower Extremity Injury Diagnosis, Testing, and Treatment
6.0 Therapeutic Procedures – Non-Operative
7.0 Therapeutic Procedures – Operative
PART A CARPAL TUNNEL SYNDROME GUIDELINES
Pursuant
to 19 Del.C. §2322C, health care practice guidelines have been adopted
and recommended by the Health Care Advisory Panel to guide utilization of
health care treatments in workers' compensation including, but not limited to,
care provided for the treatment of employees by or under the supervision of a
licensed health care provider, prescription drug utilization, inpatient
hospitalization and length of stay, diagnostic testing, physical therapy,
chiropractic care and palliative care.
The health care practice guidelines apply to all treatments provided
after the effective date of the regulation adopted by the Department of Labor,
May 23, 2008, and regardless of the date of injury. The guidelines are, to the
extent permitted by the most current medical science or applicable science,
based on well-documented scientific research concerning efficacious treatment
for injuries and occupational disease.
To the extent that well-documented scientific research regarding the
above is not available at the time of adoption of the guidelines, or is not
available at the time of any revision to the guidelines, the guidelines have
been and will be based upon the best available information concerning national
consensus regarding best health care practices in the relevant health care
community.
The guidelines, to the extent practical and
consistent with the Act, address treatment of those physical conditions which
occur with the greatest frequency, or which require the most expensive
treatments, for work-related injuries based upon currently available Delaware
data.
Services rendered by any health care provider
certified pursuant to 19 Del.C. §2322D(a) to provide treatment or
services for injured employees shall be presumed, in the absence of contrary
evidence, to be reasonable and necessary if such treatment and/or services
conform to the most current version of the Delaware health care practice
guidelines.
Services rendered outside the Guidelines and/or
variation in treatment recommendations from the Guidelines may represent
acceptable medical care, be considered reasonable and necessary treatment and,
therefore, determined to be compensable, absent evidence to the contrary, and
may be payable in accordance with the Fee Schedule and Statute, accordingly.
Services provided by any health care provider that
is not certified pursuant to 19 Del.C. §2322D(a) shall not be presumed
reasonable and necessary unless such services are pre-authorized by the
employer or insurance carrier, subject to the exception set forth in 19 Del.C.
§2322D(b).
Treatment of conditions unrelated to the injuries
sustained in an industrial accident may be denied as unauthorized if the
treatment is directed toward the non-industrial condition, unless the treatment
of the unrelated injury is rendered necessary as a result of the industrial
accident.
The Health Care Advisory Panel and Department of
Labor recognized that acceptable medical practice may include deviations from
these Guidelines, as individual cases dictate. Therefore, these Guidelines are
not relevant as evidence of a provider's legal standard of professional care.
In accordance with the
requirements of the Act, the development of the health care guidelines has been
directed by a predominantly medical or other health professional panel, with
recommendations then made to the Health Care Advisory Panel.
The principles summarized in this section are key
to the intended implementation of all Division of Workers’ Compensation
guidelines and critical to the reader’s application of the guidelines in this
document.
2.1 EDUCATION of the patient and family, as well as the
employer, insurer, policy makers and the community should be the primary
emphasis in the treatment of CTS and disability. Currently, practitioners often
think of education last, after medications, manual therapy and surgery. Practitioners must develop and implement an
effective strategy and skills to educate patients, employers, insurance
systems, policy makers and the community as a whole. An education-based
paradigm should always start with inexpensive communication providing
reassuring information to the patient.
More in-depth education currently exists within a treatment regime
employing functional restorative and innovative programs of prevention and
rehabilitation. No treatment plan is
complete without addressing issues of individual and/or group patient education
as a means of facilitating self-management of symptoms and prevention.
2.2 TREATMENT PARAMETER
time frames for specific interventions commence once treatments have
been initiated, not on the date of injury.
Obviously, duration will be impacted by patient compliance, as well
ascomorbitities and availability of services.
Clinical judgment may substantiate the need to accelerate or
deceleratemodify the time framestotal number of visits discussed in this
document. The majority of injured workers with Capal Tunnel Syndrome often will
achieve resolution of their condition within 12 to 56 visits (Guide To Physical
Therapy Practice – Second Edition). It
is anticipated that most injured workers will not require the maximum number of
visits described in these guidelines. They are designed to be a ceiling and
care extending beyond the maximum allowed visits may warrant utilization
review.
2.3 ACTIVE INTERVENTIONS emphasizing patient
responsibility, such as therapeutic exercise and/or functional treatment, are
generally emphasized over passive modalities, especially as treatment
progresses. Generally, passive
interventions are viewed as a means to facilitate progress in an active
rehabilitation program with concomitant attainment of objective functional
gains. All rehabilitation programs must
incorporate “Active Interventions” no later than three weeks after the onset of
treatment. Reimbursement for passive modalities
only after the first three weeks of treatment without clear evidence of Active
Interventions will require supportive documentation.
2.4 ACTIVE THERAPEUTIC EXERCISE PROGRAM Exercise program
goals should incorporate patient strength, endurance, flexibility,
coordination, and education. This
includes functional application in vocational or community settings.
2.5 POSITIVE PATIENT RESPONSE Positive results are defined
primarily as functional gains that can be objectively measured. Objective functional gains include, but are
not limited to, positional tolerances, range-of-motion, strength, endurance,
activities of daily living, cognition, behavior, and efficiency/ velocity measures
that can be quantified. Subjective reports
of pain and function should be considered and given relative weight when the
pain has anatomic and physiologic correlation.
Anatomic correlation must be based on objective findings.
2.6 RE-EVALUATE TREATMENT EVERY 3 TO 4 WEEKS If a given
treatment or modality is not producing positive results within 3 to 4 weeks,
the treatment should be either modified or discontinued. Reconsideration of
diagnosis should also occur in the event of poor response to a seemingly
rational intervention.
2.7 SURGICAL INTERVENTIONS Surgery should be contemplated
within the context of expected functional outcome and not purely for the
purpose of pain relief. The concept of
“cure” with respect to surgical treatment by itself is generally a misnomer. All operative interventions must be based
upon positive correlation of clinical findings, clinical course and diagnostic
tests. A comprehensive assimilation of
these factors must lead to a specific diagnosis with positive identification of
pathologic conditions.
2.8 SIX-MONTH TIME-FRAME The prognosis drops precipitously
for returning an injured worker to work once he/she has been temporarily
totally disabled for more than six months.
The emphasis within these guidelines is to move patients along a continuum
of care and return-to-work within a six-month time frame, whenever
possible. It is important to note that
time frames may not be pertinent to injuries that do not involve work-time loss
or are not occupationally related.
2.9 RETURN-TO-WORK is therapeutic, assuming the work is
not likely to aggravate the basic problem or increase long-term pain. The practitioner must provide specific
physical limitations per the Physician’s Form. The following physical
limitations should be considered and modified as recommended: lifting, pushing, pulling, crouching, walking,
using stairs, bending at the waist, awkward and/or sustained postures,
tolerance for sitting or standing, hot and cold environments, data entry and
other repetitive motion tasks, sustained grip, tool usage and vibration
factors. Even if there is residual
chronic pain, return-to-work is not necessarily contraindicated.The
practitioner should understand all of the physical demands of
the patient’s job position before returning the patient to full
duty and should receive clarification of the patient’s job
duties.
2.10 DELAYED RECOVERY Strongly consider a psychological
evaluation, if not previously provided, as well as initiating interdisciplinary
rehabilitation treatment and vocational goal setting, for those patients who
are failing to make expected progress 6 to 12 weeks after an injury. The Division recognizes that 3 to 10% of all
industrially injured patients will not recover within the timelines outlined in
this document despite optimal care. Such individuals may require
treatments beyond the limits discussed within this document, but
such treatment will require clear documentation by the
authorized treating practitioner focusing on objective
functional gains afforded by further treatment and impact upon
prognosis.
2.11 GUIDELINE RECOMMENDATIONS AND INCLUSION OF MEDICAL EVIDENCE Guidelines are recommendations based on available evidence
and/or consensus recommendations. Those procedures considered
inappropriate, unreasonable, or unnecessary are designated in
the guideline as being “not recommended.”
The remainder of this
document should be interpreted within the parameters of these guideline
principles that may lead to more optimal medical and functional outcomes for
injured workers.
Carpal tunnel syndrome
(CTS) is one of the most common mononeuropathies (a disorder involving only a
single nerve). The median nerve is extremely vulnerable to compression and
injury in the region of the wrist and palm. In this area, the nerve is bounded
by the wrist bones and the transverse carpal ligament. The most common site of
compression is at the proximal edge of the flexor retinaculum (an area near the
crease of the wrist). There is often a
myofascial component in the patient's presentation. This should be considered
when proceeding with the diagnostic workup and therapeutic intervention.
Studies have repeatedly
confirmed that the diagnosis cannot be made based on any single historical
factor or physical examination finding. Electrodiagnostic tests may be negative
in surgically confirmed cases. Conversely, electrodiagnostic testing may be
positive in asymptomatic individuals. The diagnosis of CTS, therefore, remains
a clinical diagnosis based on a preponderance of supportive findings.
Classic findings of CTS
include subjective numbness or dysesthesias confined to the median nerve
distribution, worsening of symptoms at night, and positive exam findings.
Please refer to other appropriate upper extremity guidelines as necessary.
4.1 INTRODUCTION The two standard procedures that are to
be utilized when initially evaluating a work-
related carpal tunnel complaint are History Taking,
and Physical Examination. History-taking and Physical Examination are generally
accepted, well-established, and widely used procedures which establish the
foundation/basis for and dictate all ensuing stages of diagnostic and
therapeutic procedures. When findings of clinical evaluation and those of other
diagnostic procedures do not complement each other, the objective clinical
findings should have preference.
4.2 HISTORY
4.2.1 Description of symptoms - should address
at least the following:
4.2.1.1 Numbness, tingling, and/or burning of
the hand involving the distal median nerve distribution; however, distribution
of the sensory symptoms may vary considerably between individuals. Although the
classic median nerve distribution is to the palmar aspect of the thumb, the
index finger, the middle finger and radial half of the ring finger, patients
may report symptoms in any or all of the fingers. The Katz Hand diagram (see
Fig. 1) may be useful in documenting the distribution of symptoms; the classic
pattern of carpal tunnel affects at least two of the first three digits and
does not involve dorsal and palmar aspects of the hand. A probable pattern
involves the palmar but not dorsal aspect of the hand (excluding digits).
4.2.1.2 Nocturnal symptoms frequently disrupt
sleep and consist of paresthesias and/or pain in the hand and/or arm.
4.2.1.3 Pain in the wrist occurs frequently and
may even occur in the forearm, elbow or shoulder. While proximal pain is not
uncommon, its presence warrants evaluation for other pathology in the cervical
spine, shoulder and upper extremity.
4.2.1.4 Shaking the symptomatic hand to relieve
symptoms may be reported.
4.2.1.5 Clumsiness of the hand or dropping
objects is often reported, but may not be present early in the course.
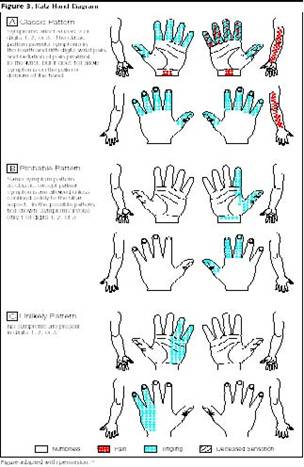
Figure 1 – Katz Hand Diagram Used with permission.
JAMA 2000; 283 (23): 3110-17. Copyrighted 2000, American Medical Association.
4.2.2 Identification of Occupational
Risk Factors: Job title alone is not sufficient information. The clinician
is responsible for documenting specific information regarding repetition, force
and other risk factors, as listed in the table entitled, ‘Risk Factors
Associated with CTS’- Table 2. A job site evaluation may be required.
4.2.3 Demographics: Age, hand
dominance, gender, etc.
4.2.4 Past Medical History and Review of
Systems: A study of CTS patients showed a 33% prevalence of related
disease. Risk factors for CTS include female gender; obesity; Native American,
Hispanic, or Black heritage, and certain medical conditions:
4.2.4.1 Pregnancy
4.2.4.2 Arthropathies including connective
tissue disorders, rheumatoid arthritis, systemic lupus erythematosus, gout,
osteoarthritis and spondyloarthropathy
4.2.4.3 Colles’ fracture or other acute trauma
4.2.4.4 Amyloidosis
4.2.4.5 Hypothyroidism, especially in older
females
4.2.4.6 Diabetes mellitus, including family
history or gestational diabetes
4.2.4.7 Acromegaly
4.2.4.8 Use of corticosteroids or estrogens
4.2.4.9 Vitamin B6 deficiency
4.2.5 Activities of Daily Living (ADLs):
include such activities as self care and personal hygiene, communication,
ambulation, attaining all normal living postures, travel, non-specialized hand
activities, sexual function, sleep, and social and recreational activities.
Specific movements in this category include pinching or grasping
keys/pens/other small objects, grasping telephone receivers or cups or other
similar-sized objects, and opening jars. The quality of these activities is
judged by their independence, appropriateness, and effectiveness. Assess not
simply the number of restricted activities but the overall degree of
restriction or combination of restrictions.
4.2.6 Avocational Activities: Information
must be obtained regarding sports, recreational, and other avocational
activities that might contribute to or be impacted by CTD development.
Activities such as hand-operated video games, crocheting/needlepoint, home
computer operation, golf, racquet sports, bowling, and gardening are included
in this category.
4.2.7 Social History: Exercise
habits, alcohol consumption, and psychosocial factors.
4.3 PHYSICAL EXAMINATION Please refer to Table 1 for
respective sensitivities and specificities for findings used to diagnose CTS
(a-f).
4.3.1 Sensory loss to pinprick, light
touch, two-point discrimination or Semmes-Weinstein Monofilament tests in a
median nerve distribution may occur
4.3.2 Thenar atrophy may appear, but
usually late in the course
4.3.3 Weakness of the abductor pollicis
brevis may be present
4.3.4 Phalen’s / Reverse Phalen’s signs may
be positive
4.3.5 Tinel’s sign over the carpal tunnel
may be positive
4.3.6 Closed Fist test – holding fist
closed for 60 seconds reproduces median nerve paresthesia
4.3.7 Evaluation of the contralateral wrist
is recommended due to the frequency of bilateral involvement
4.3.8 Evaluation of the proximal upper
extremity and cervical spine for other disorders including cervical
radiculopathy, thoracic outlet syndrome, other peripheral neuropathies, and
other musculoskeletal disorders
4.3.9 Signs of underlying medical disorders
associated with CTS, e.g., diabetes mellitus, arthropathy, and hypothyroidism
4.3.10 Myofascial findings requiring treatment
may present in soft tissue areas near other CTD pathology, and should be
documented. Refer to the Division’s Cumulative Trauma Disorder Medical
Treatment Guidelines.
Table 1: Sensitivities and Specificities and
Evidence Level for Physical Examination findings
TITLE 19 LABOR
DELAWARE ADMINISTRATIVE CODE
|
|
Procedure
|
Sensitivity (%)
|
Specificity (%)
|
Validity
|
|
1. Sensory testing
|
|
|
|
|
Hypesthesia
|
15-51
|
85-93
|
Good
|
|
Katz Hand Diagram
|
62-89
|
73-88
|
Good
|
|
Two-point discrimination
|
22-33
|
81-100
|
Some
|
|
Semmes-Weinstein
|
52-91
|
59-80
|
Some
|
|
Vibration
|
20-61
|
71-81
|
None
|
|
2. Phalen’s
|
51-88
|
32-86
|
Some
|
|
3. Tinel’s
|
25-73
|
55-94
|
Some
|
|
4. Carpal tunnel compression
|
28-87
|
33-95
|
Some
|
|
5. Thenar atrophy
|
3-28
|
82-100
|
Good
|
|
Abductor pollicis brevis weakness
|
63-66
|
62-66
|
Good
|
|
6. Closed fist test
|
61
|
92
|
Some
|
|
7. Tourniquet test
|
16-65
|
36-87
|
None
|
4.4 RISK FACTORS A
critical review of epidemiologic literature identified a number of physical
exposures associated with CTS. For example, trauma and fractures of the hand
and wrist may result in CTS. Other physical exposures considered risk factors
include: repetition, force, vibration, pinching and gripping, and cold
environment. When workers are exposed to several risk factors simultaneously,
there is an increased likelihood of CTS. Not all risk factors have been
extensively studied. Exposure to cold environment, for example, was not
examined independently; however, there is good evidence that combined with
other risk factors cold environment increases the likelihood of a CTS. Table 2 at the end of this section entitled,
"Risk Factors Associated CTS," summarizes the results of currently
available literature.
No single epidemiologic study will fulfill
all criteria for causality. The clinician must recognize that currently
available epidemiologic data is based on population results, and that
individual variability lies outside the scope of these studies. Many published
studies are limited in design and methodology, and, thus, preclude conclusive
results. Most studies' limitations tend to attenuate, rather than inflate,
associations between workplace exposures and CTS.
These guidelines are based on current
epidemiologic knowledge. As with any scientific work, the guidelines are
expected to change with advancing knowledge. The clinician should remain
flexible and incorporate new information revealed in future studies.
Table 2: Risk Factors Associated with Carpal Tunnel Syndrome
|
Diagnosis
|
Strong Evidence
|
Good evidence
|
Some evidence
|
Insufficient or
conflicting evidence
|
|
|
|
|
|
|
|
Carpal Tunnel Syndrome
|
Combination of high
exertional force (Varied from greater than 6 kg) and high repetition (work
cycles less than 30 sec or greater than 50% of cycle time performing same
task, length of shortest task less than 10 sec).
|
Repetition or force independe
ntly, use of vibration hand tools.
|
Wrist ulnar deviation and
extension.
|
Pinch/grip, keyboarding.
|
4.5 LABORATORY TESTS
Laboratory tests are generally accepted, well-established, and widely used
procedures. Patients should be carefully screened at the initial exam for signs
or symptoms of diabetes, hypothyroidism, arthritis, and related inflammatory
diseases. The presence of concurrent disease does not negate work-relatedness
of any specific case. When a patient's history and physical examination suggest
infection, metabolic or endocrinologic disorders, tumorous conditions, systemic
musculoskeletal disorders (e.g., rheumatoid arthritis), or potential problems
related to prescription of medication (e.g., renal disease and nonsteroidal
anti-inflammatory medications), then laboratory tests, including, but not
limited to, the following can provide useful diagnostic information:
4.5.1 Serum
rheumatoid factor and Antinuclear Antigen (ANA) for rheumatoid work-up;
4.5.2 Thyroid
Stimulating Hormone (TSH) for hypothyroidism;
4.5.3 Fasting
glucose - recommended for obese men and women over 40 years of age, patients
with a history of family diabetes, those from high-risk ethnic groups, and with
a previous history of impaired glucose tolerance. A fasting blood glucose greater
than 125mg/dl is diagnostic for diabetes. Urine dipstick positive for glucose
is a specific but not sensitive screening test. Quantitative urine glucose is
sensitive and specific in high-risk populations;
4.5.4 Serum
protein electrophoresis;
4.5.5 Sedimentation
rate, nonspecific, but elevated in infection, neoplastic conditions and
rheumatoid arthritis;
4.5.6 Serum
calcium, phosphorus, uric acid, alkaline and acid phosphatase for metabolic,
endocrine and neoplastic conditions;
4.5.7 Complete
Blood Count (CBC), liver and kidney function profiles for metabolic or
endocrine disorders or for adverse effects of various medications;
4.5.8 Bacteriological
(microorganism) work-up for wound, blood and tissue;
4.5.9 Serum
B6 – routine screening is not recommended due to the fact that vitamin B6
supplementation has not been proven to affect the course of carpal tunnel
syndrome. However, it may be appropriate for patients on medications that
interfere with the effects of vitamin B6, or for those with significant
nutritional problems.
The Department recommends the above
diagnostic procedures be considered, at least initially, the responsibility of
the workers' compensation carrier to ensure that an accurate diagnosis and
treatment plan can be established.
5.1 ELECTRODIAGNOSTIC
(EDX) STUDIES are well established and widely accepted for evaluation of
patients suspected of having CTS. The results are highly sensitive and specific
for the diagnosis. Studies may confirm the diagnosis or direct the examiner to
alternative disorders. Studies require clinical correlation due to the
occurrence of false positive and false negative results. Symptoms of CTS may occur with normal EDX
studies, especially early in the clinical course.
EDX findings in CTS reflect slowing of
median motor and sensory conduction across the carpal tunnel region due to
demyelination. Axonal loss, when present, is demonstrated by needle
electromyography in median nerve-supplied thenar muscles. Findings include
fibrillations, fasciculations, neurogenic recruitment and polyphasic units
(reinnervation).
5.1.1 Needle
electromyography of a sample of muscles innervated by the C5 to T1 spinal
roots, including a thenar muscle innervated by the median nerve of the
symptomatic limb, is frequently required.
5.1.2 The
following EDX studies are not recommended to confirm a clinical diagnosis of
CTS:
5.1.2.1 Low
sensitivity and specificity compared to other EDX studies: multiple median F
wave parameters, median motor nerve residual latency, and sympathetic skin
response
5.1.2.2 Investigational
studies: evaluation of the effect on median NCS of limb ischemia, dynamic hand
exercises, and brief or sustained wrist positioning
5.1.3 To
assure accurate testing, temperature should be maintained at 30-34C preferably
recorded from the hand/digits. For temperature below 30C the hand should be
warmed.
5.1.4 All
studies must include normative values for their laboratories.
5.1.5 Positive
Findings – Any of these nerve conduction study findings must be accompanied by
median nerve symptoms to establish the diagnosis.
5.1.5.1 Slowing
of median distal sensory and/or motor conduction through the carpal tunnel
region
5.1.5.2 Electromyographic
changes in the median thenar muscles in the absence of proximal abnormalities
5.1.6 Because
laboratories establish their own norms, a degree of variability from the
suggested guideline values is acceptable.
5.1.7 In
all cases, normative values are to be provided with the neurodiagnostic
evaluation.
5.1.8 Suggested
grading scheme by electrodiagnostic criteria for writing a consultation or
report may be:
5.1.8.1 Mild
CTS-prolonged (relative or absolute) median sensory or mixed action potential
distal latency (orthodromic, antidromic, or palmar).
5.1.8.2 Moderate
CTS-abnormal median sensory latencies as above, and prolongation (relative or
absolute) of median motor distal latency.
5.1.8.3 Severe
CTS-prolonged median motor and sensory distal latencies, with either absent
sensory or palmar potential, or low amplitude or absent thenar motor action
potential. Needle examination reveals evidence of acute and chronic denervation
with axonal loss.
5.1.9 Frequency
of Studies/Maximum Number of Studies:
5.1.9.1 Indications for Initial Testing:
5.1.9.1.1 Patients
who do not improve symptomatically or functionally with conservative measures
for carpal tunnel syndrome over a 3-4 week period
5.1.9.1.2 Patients
in whom the diagnosis is in question
5.1.9.1.3 Patients
for whom surgery is contemplated
5.1.9.1.4 To
rule out other nerve entrapments or a radiculopathy
5.1.9.2 Repeated studies may be performed:
5.1.9.2.1 To
determine disease progression. 8-12 weeks is most useful when the initial
studies were normal and CTS is still suspected
5.1.9.2.2 For
inadequate improvement with non-surgical treatment for 8-12 weeks
5.1.9.2.3 For
persistent or recurrent symptoms following carpal tunnel release, post-op 3-6
months, unless an earlier evaluation is required by the surgeon
5.2
IMAGING STUDIES
5.2.1 Radiographic
Imaging: Not generally required for most CTS diagnoses. However, it may be
necessary to rule out other pathology in the cervical spine, shoulder, elbow,
wrist or hand. Wrist and elbow radiographs would detect degenerative joint
disease, particularly scapholunate dissociation and thumb carpometacarpal
abnormalities which occasionally occur with CTS.
5.2.2 Magnetic
Resonance Imaging (MRI): Considered experimental and not recommended for
diagnosis of Carpal Tunnel Syndrome. Trained neuroradiologists have not
identified a single MRI parameter that is highly sensitive and specific. MRI is
less accurate than standard electrodiagnostic testing, and its use as a
diagnostic tool is not recommended.
5.2.3 Sonography:
This tool has not been sufficiently studied to define its diagnostic
performance relative to electrodiagnostic studies. It is not a widely applied
test. Sonography may detect synovial thickening in CTS caused by rheumatoid
arthritis. It may be useful if space-occupying lesions, such as, lipomas,
hemangiomas, fibromas, and ganglion cysts, are suspected. Its routine use in
CTS is not recommended.
5.3 ADJUNCTIVE TESTING Clinical
indications for the use of tests and measurements are predicated on the history
and systems review findings, signs observed on physical examination, and
information derived from other sources and records. They are not designed to be
the definitive indicator of dysfunction.
5.3.1 Electromyography:
is a generally accepted, well-established procedure. It is indicated when acute and/or chronic
neurogenic changes in the thenar eminence are associated with the conduction
abnormalities discussed above.
5.3.2 Electroneurometer:
May serve as a diagnostic tool as it helps to detect early distal
sensorineural impairment.
5.3.3 Portable
Automated Electrodiagnostic Device: Measures distal median nerve motor
latency and F-wave latency at the wrist and has been tested in one research
setting. It performed well in this setting following extensive calibration of
the device. Motor nerve latency compared favorably with conventional
electrodiagnostic testing, but F-wave latency added little to diagnostic
accuracy. It remains an investigational instrument whose performance in a
primary care setting is as yet not established, and is not recommended as a
substitute for conventional electrodiagnostic testing in clinical
decision-making.
5.3.4 Quantitative
Sensory Testing (QST): May be used as a screening tool in clinical settings
pre- and post-operatively. Results of tests and measurements of sensory
integrity are integrated with the history and systems review findings and the
results of other tests and measures. QST has been divided into two types of
testing:
5.3.4.1 Threshold
tests measure topognosis, the ability to exactly localize a cutaneous
sensation, and pallesthesia, the ability to sense mechanical using vibration
discrimination testing (quickly adapting fibers); Semmes-Wienstein monofilament
testing (slowly adapting fibers);
5.3.4.2 Density
Tests also measure topognosis and pallesthesia using static two-point
discrimination (slowly adapting fibers); moving two-point discrimination
(quickly adapting fibers).
5.3.5 Pinch
and Grip Strength Measurements: May be accepted as a diagnostic tool for
CTS. Strength is defined as the muscle force exerted by a muscle or group of
muscles to overcome a resistance under a specific set of circumstances. Pain,
the perception of pain secondary to abnormal sensory feedback, and/or the
presence of abnormal sensory feedback affecting the sensation of the power used
in grip/pinch may cause a decrease in the force. When all five handle settings
of the dynamometer are used, a bell-shaped curve, reflecting maximum strength
at the most comfortable handle setting, should be present. These measures
provide a method for quantifying strength that can be used to follow a
patient’s progress and to assess response to therapy. In the absence of a bell-shaped
curve, clinical reassessment is indicated.
5.3.6 Laboratory
Tests In one study of carpal tunnel patients seen by specialists, 9% of
patients were diagnosed with diabetes, 7% with hypothyroidism, and 15% with chronic
inflammatory disease including spondyloarthropathy, arthritis, and systemic
lupus erythematosis. Up to two thirds of the patients were not aware of their
concurrent disease. Estimates of the prevalence of hypothyroidism in the
general population vary widely, but data collected from the Colorado Thyroid
Disease Prevalence Study revealed subclinical hypothyroidism in 8.5% of
participants not taking thyroid medication. The prevalence of chronic joint
symptoms in the Behavioral Risk Factor Surveillance System (BRFSS) from the
Centers for Disease Control (CDC) was 12.3%. If after 2-3 weeks, the patient is
not improving the physician should strongly consider the following laboratory
studies: thyroid function studies, rheumatoid screens, chemical panels, and others,
if clinically indicated.
Laboratory testing may be required
periodically to monitor patients on chronic medications.
Before initiation of any therapeutic
procedure, the authorized treating provider, employer, and insurer
must
consider these important issues in the care of the injured worker.
First, patients undergoing therapeutic procedure(s) should be released or
returned to modified or
restricted duty during their rehabilitation at the earliest appropriate
time. Refer to “Return-to-Work” in
this section for detailed information.
Second, cessation and/or review of treatment
modalities should be undertaken when no further significant subjective or
objective improvement in the patient’s condition is noted. If patients are not responding within the
recommended duration periods, alternative treatment interventions, further
diagnostic studies or consultations should be pursued.
Third, providers should provide and document
education to the patient. No treatment plan is complete without addressing
issues of individual and/or group patient education as a means of facilitating
self-management of symptoms.
In cases where a patient is unable to attend
an outpatient center, home therapy may be necessary. Home therapy may include
active and passive therapeutic procedures as well as other modalities to assist
in alleviating pain, swelling, and abnormal muscle tone. Home therapy is usually of short duration and
continues until the patient is able to tolerate coming to an outpatient center.
Non-operative treatment procedures for CTS
can be divided into two groups: conservative care and rehabilitation. Conservative care is treatment applied to a
problem in which spontaneous improvement is expected in 90% of the cases within
three months. It is usually provided
during the tissue-healing phase and lasts no more than six months, and often
considerably less. Rehabilitation is
treatment applied to a more chronic and complex problem in a patient with
de-conditioning and disability. It is provided during the period after tissue
healing to obtain maximal medical recovery.
Treatment modalities may be utilized sequentially or concomitantly
depending on chronicity and complexity of the problem, and treatment plans
should always be based on a diagnosis utilizing appropriate diagnostic
procedures.
The following procedures are listed in
alphabetical order.
6.1 ACUPUNCTURE
is an accepted and widely used procedure for the relief of pain and inflammation. The exact mode of action is only partially understood. Western medicine studies suggest that acupuncture stimulates the nervous system at the level of the brain, promotes deep relaxation, and affects the release of neurotransmitters. Acupuncture is commonly used as an alternative or in addition to traditional Western pharmaceuticals. While it is commonly used when pain medication is reduced or not tolerated, it may be used as an adjunct to physical rehabilitation and/or surgical intervention to hasten the return of functional activity. Acupuncture should be performed by MD, DO, DC with appropriate training; or a licensed acupuncturist.
6.1.1 Definition:
Acupuncture is the insertion and removal of filiform needles to stimulate
acupoints (acupuncture points). Needles
may be inserted, manipulated, and retained for a period of time. Acupuncture
can be used to reduce pain, reduce inflammation, increase blood flow, increase
range of motion, decrease the side effect of medication-induced nausea, promote
relaxation in an anxious patient, and reduce muscle spasm.
Indications include joint pain, joint
stiffness, soft tissue pain and inflammation, paresthesia, post-surgical pain
relief, muscle spasm, and scar tissue pain.
Time to produce
effect: 3 to 6 treatments
Frequency: 1 to 3 times per week
Course duration: 14 treatments
6.1.2 Acupuncture
with Electrical Stimulation: is the use of electrical current (micro-
amperage or milli-amperage) on the needles at the acupuncture site. It is used to increase effectiveness of the
needles by continuous stimulation of the acupoint. Physiological effects
(depending on location and settings) can include endorphin release for pain
relief, reduction of inflammation, increased blood circulation, analgesia
through interruption of pain stimulus, and muscle relaxation.
It is indicated to treat chronic pain
conditions, radiating pain along a nerve pathway, muscle spasm, inflammation,
scar tissue pain, and pain located in multiple sites.
Time to produce
effect: 3 to 6 treatments
Frequency: 1 to 3 times per week
Course duration: 14 treatments
6.1.3 Other
Acupuncture Modalities: Acupuncture treatment is based on individual
patient needs and therefore treatment may include a combination of procedures
to enhance treatment effect. Other procedures may include the use of heat, soft
tissue manipulation/massage, and exercise.
Refer to sections F 12 and 13 Active Therapy and Passive Therapy for a
description of these adjunctive acupuncture modalities.
Time to produce
effect: 3 to 6 treatments
Frequency: 1 to 3 times per week
• Course duration:
14 treatments Any of the above acupuncture treatments may extend longer
if objective functional gains can be documented or when symptomatic benefits
facilitate progression in the patient’s treatment program. Treatment beyond 14 treatments may be
documented with respect to need and
ability to facilitate positive symptomatic
or functional gains. Such care should be re-evaluated and documented with each
series of treatments.
6.2 BIOFEEDBACK
is a form of behavioral medicine that helps patients learn self-awareness
and self-regulation skills for the purpose of gaining greater control of their
physiology, such as muscle activity, brain waves, and measures of autonomic
nervous system activity. Electronic
instrumentation is used to monitor the targeted physiology and then displayed
or fed back to the patient visually, auditorially or tactilely, with coaching
by a biofeedback specialist. Biofeedback
is provided by clinicians certified in biofeedback and/or who have documented
specialized education, advanced training, or direct or supervised experience
qualifying them to provide the specialized treatment needed (e.g., surface EMG,
EEG, or other).
Treatment is individualized to the patient’s
work-related diagnosis and needs. Home
practice of skills is required for mastery and may be facilitated by the use of
home training tapes. The ultimate goal in biofeedback treatment is normalizing
the physiology to the pre-injury status to the extent possible and involves
transfer of learned skills to the workplace and daily life. Candidates for
biofeedback therapy or training must be motivated to learn and practice
biofeedback and self-regulation techniques.
Indications for biofeedback include
individuals who are suffering from musculoskeletal injury where muscle
dysfunction or other physiological indicators of excessive or prolonged stress
response affects and/or delays recovery.
Other applications include training to improve self-management of
emotional stress/pain responses such as anxiety, depression, anger, sleep
disturbance, and other central and autonomic nervous system imbalances. Biofeedback is often utilized along with
other treatment modalities.
Time to produce
effect: 3 to 4 sessions
Frequency: 1 to 2 times per week
Maximum
duration: 10 to 12 sessions. Treatment beyond 12 sessions must be
documented with respect to need, expectation, and ability to facilitate
positive symptomatic or functional gains.
6.3 INJECTIONS-THERAPEUTIC
Steroids Injections - Beneficial effects of injections are well-established,
but generally considered to be temporary. Recurrence of symptoms is frequent.
It is not clear whether or not injections slow progression of electrodiagnostic
changes. Therefore, although symptoms may be temporarily improved, nerve damage
may be progressing. When motor changes are present, surgery is preferred over
injections.
Time to produce
effect: 2-5 days
Frequency: every 6-8 weeks
Optimum number: 2
injections
• Maximum number:
3 injections in 6 months If following the first injection, symptomatic
relief is followed by recurrent symptoms, the decision to
perform a second injection must be weighed
against alternative treatments such as surgery.
Surgery may give more definitive relief of symptoms.
6.4 JOB
SITE ALTERATION Early evaluation and training of body mechanics and other
ergonomic factors are essential for every injured worker and should be done by
a qualified individual. In some cases, this requires a job site evaluation.
Some evidence supports alteration of the job site in the early treatment of
Carpal Tunnel Syndrome (CTS). There is no single factor or combination of
factors that is proven to prevent or ameliorate CTS, but a combination of ergonomic
and psychosocial factors is generally considered to be important. Physical
factors that may be considered include use of force, repetition, awkward
positions, upper extremity vibration, cold environment, and contact pressure on
the carpal tunnel. Psychosocial factors to be considered include pacing, degree
of control over job duties, perception of job stress, and supervisory support.
The job analysis and modification should
include input from the employee, employer, and ergonomist or other professional
familiar with work place evaluation. The employee must be observed performing
all job functions in order for the job site analysis to be valid. Periodic
follow-up is recommended to evaluate effectiveness of the intervention and need
for additional ergonomic changes.
6.4.1 Ergonomic
changes: should be made to modify the hazards identified. In addition
workers should be counseled to vary tasks throughout the day whenever possible.
Occupational Safety and Health Administration (OSHA) suggests that workers who
perform repetitive tasks, including keyboarding, take 15-30 second breaks every
10 to 20 minutes, or 5-minute breaks every hour. Mini breaks should include
stretching exercises.
6.4.2 Interventions:
should consider engineering controls, e.g., mechanizing the task, changing the
tool used, or adjusting the work site, or administrative controls, e.g.,
adjusting the time an individual performs the task.
6.4.3 Seating
Description: The following description may aid in evaluating seated work
positions: The head should incline only slightly forward, and if a monitor is
used, there should be 18-24 inches of viewing distance with no glare. Arms
should rest naturally, with forearms parallel to the floor, elbows at the
sides, and wrists straight or minimally extended. The back must be properly
supported by a chair, which allows change in position and backrest adjustment.
There must be good knee and legroom, with the feet resting comfortably on the
floor or footrest. Tools should be within easy reach, and twisting or bending
should be avoided.
6.4.4 Job
Hazard Checklist: The following Table 3 is adopted from Washington State’s
job hazard checklist, and may be used as a generally accepted guide for
identifying job duties which may pose ergonomic hazards. The fact that an
ergonomic hazard exists at a specific job, or is suggested in the table, does
not establish a causal relationship between the job and the individual with a
musculoskeletal injury. However, when an individual has a work-related injury
and ergonomic hazards exist that affect the injury, appropriate job
modifications should be made. Proper
correction of hazards may prevent future injuries to others, as well as aid in
the recovery of the injured worker.
Table 3: Identifying Job Duties Which May Pose Ergonomic Hazards
TITLE
19 LABOR
DELAWARE ADMINISTRATIVE CODE
|
Type of Job Duty
|
Hours per Day
|
|
Pinching an unsupported
object(s) weighing 2 lbs or more per hand, or pinching with a force of 4 lbs
or more per hand (comparable to pinching a half a ream of paper): 1. Highly
repetitive motion 2. Palmar flexion greater than 30 degrees, dorsiflexion greater
than 45 degrees, or radial deviation greater than 30 degrees 3. No other risk
factors
|
More than 3 hours total/day
More than 4 hours total/day
|
|
Gripping an unsupported
object(s) weighing 10 lbs or more/hand, or gripping with a force of 10 lbs or
more/hand (comparable to clamping light duty automotive jumper cables onto a
battery): *Handles should be rounded and soft, with at least 1-2.5” in
diameter grips at least 5” long. 1. Highly repetitive motion 2. Palmar
flexion greater than 30 degrees, dorsiflexion greater than 45 degrees, or
radial deviation greater than 30 degrees 3. No other risk factors
|
More than 3 hours total/day
More than 4 hours total/day
|
|
Repetitive Motion (using the
same motion with little or no variation every few seconds), excluding keying
activities: 1. High, forceful exertions with the hands, with palmar flexion
greater than 30 degrees, dorsiflexion greater than 45 degrees, or radial
deviation greater than 30 degrees 2. No other risk factors
|
More than 2 hours total/day
More than 6 hours total/day
|
|
Intensive Keying: 1. Palmar
flexion greater than 30 degrees, dorsiflexion greater than 45 degrees, or
radial deviation greater than 30 degrees 2. No other risk factors
|
More than 4 hours total/day
More than 7 hours total/day
|
|
Repeated Impact: 1. Using the
hand (heel/base of palm) as a hammer more than once/minute
|
More than 2 hours total/day
|
TITLE
19 LABOR
DELAWARE ADMINISTRATIVE CODE
|
Vibration:
|
|
|
Two determinants of the
tolerability of segmental vibration of the hand are the
|
|
|
frequency and the
acceleration of the motion of the vibrating tool, with lower
|
|
|
frequencies being more poorly
tolerated at a given level of imposed acceleration,
|
|
|
expressed below in multiples
of the acceleration due to gravity (10m/sec/sec).
|
More than 30
|
|
1. Frequency range 8-15 Hz
and acceleration 6 g
|
minutes at a time
|
|
2. Frequency range 80 Hz and
acceleration 40 g
|
|
|
3. Frequency range 250 Hz and
acceleration 250 g
|
|
|
|
More than 4 hours
|
|
4. Frequency range 8-15 Hz
and acceleration 1.5 g
|
at a time
|
|
5. Frequency range 80 Hz and
acceleration 6 g
|
|
|
6. Frequency range 250 Hz and
acceleration 20 g
|
|
6.5 MEDICATIONS including
nonsteroidal anti-inflammatory medications (NSAIDS), oral steroids, diuretics,
and pyridoxine (Vitamin B6) have not been shown to have significant long-term
beneficial effect in treating Carpal Tunnel Syndrome. Although NSAIDS are not
curative, they and other analgesics may provide symptomatic relief. All
narcotics and habituating medications should be prescribed with strict time,
quantity, and duration guidelines with a definite cessation parameter.
6.5.1 Vitamin
B6: Randomized trials have demonstrated conflicting results. Higher doses
may result in development of a toxic peripheral neuropathy. In the absence of
definitive literature showing a beneficial effect, use of Vitamin B6 cannot be
recommended.
6.5.2 Oral
Steroids: have been shown to have short-term symptomatic benefit but no
long-term functional benefit and are only rarely recommended due to possible
side effects.
6.6 OCCUPATIONAL
REHABILITATION PROGRAMS
6.6.1 Non-Interdisciplinary:
These programs are work-related, outcome-focused, individualized treatment
programs. Objectives of the program
include, but are not limited to, improvement of cardiopulmonary and
neuromusculoskeletal functions (strength, endurance, movement, flexibility,
stability, and motor control functions), patient education, and symptom
relief. The goal is for patients to gain
full or optimal function and return to work.
The service may include the time-limited use of passive modalities with
progression to achieve treatment and/or simulated/real work.
6.6.1.1 Work
Conditioning/Simulation
This program may begin once a patient is out
of the acute phase of injury and will be able
to tolerate this program.
These programs are usually initiated after
the acute phase has been completed and
offered at any time throughout the recovery
phase. Work conditioning should be initiated when imminent return of a patient
to modified or full duty is not an option, but the prognosis for returning the
patient to work at completion of the program is at least fair to good.
The need for work place simulation should be
based upon the results of a Functional Capacity Evaluation and/or Jobsite
Analysis.
Length of visit:
1 to 4 hours per day.
Frequency: 2 to 5 visits per week
Maximum
duration: 8 weeks. Participation in a program beyond six weeks
must be documented with respect to need and the ability to facilitate positive
symptomatic or functional gains.
6.6.1.2 Work
Hardening
Work Hardening is an interdisciplinary
program addressing a patient’s employability and return to work. It includes a progressive increase in the
number of hours per day that a patient completes work simulation tasks until
the patient can tolerate a full workday. This is accomplished by addressing the
medical, behavioral, physical, functional, and vocational components of
employability and return-to-work.
This can include a highly structured program
involving a team approach or can involve any of the components thereof. The interdisciplinary team should, at a
minimum, be comprised of a qualified medical director who is board certified
with documented training in occupational rehabilitation; team physicians having
experience in occupational rehabilitation; occupational therapist; physical
therapist; case manager; and psychologist. As appropriate, the team may also
include: chiropractor, RN, vocational specialist or Certified Biofeedback
Therapist.
Length of
visit: Up to 8 hours/day
Frequency: 2 to 5 visits per week
Maximum
duration: 8 weeks. Participation in a program
beyond six weeks must be documented with respect to need and the ability to
facilitate positive symptomatic or functional gains.
6.7 ORTHOTICS/IMMOBILIZATION
WITH SPLINTING is a generally accepted, well-established and widely used
therapeutic procedure. There is some evidence that splinting leads to more
improvement in symptoms and hand function than watchful waiting alone. Because
of limited patient compliance with day and night splinting in published
studies, evidence of effectiveness is limited to nocturnal splinting alone.
Splints should be loose and soft enough to maintain comfort while supporting
the wrist in a relatively neutral position. This can be accomplished using a
soft or rigid splint with a metal or plastic support. Splint comfort is critical
and may affect compliance. Although off-the-shelf splints are usually
sufficient, custom thermoplastic splints may provide better fit for certain
patients.
Splints may be effective when worn at night
or during portions of the day, depending on activities. Most studies show that
full time night splinting for a total of 4 to 6 weeks is the most effective
protocol. Depending on job activities, intermittent daytime splinting can also
be helpful. Splint use is rarely mandatory. Providers should be aware that
over-usage is counterproductive, and should counsel patients to minimize
daytime splint use in order avoid detrimental effects such as stiffness and
dependency over time.
Splinting is generally effective for milder
cases of CTS. Long-term benefit has not been established. An effect should be
seen in 2-4 weeks.
Time to produce
effect: 1-4 weeks. If, after 4 weeks, the patient has partial improvement,
continue to follow since neuropathy may worsen, even in the face of diminished
symptoms.
Frequency: Nightly. Daytime intermittent, depending on
symptoms and activities
Maximum
duration: 2 to 4 months. If symptoms
persist, consideration should be given to either repeating electrodiagnostic
studies or to more aggressive treatment.
6.8 PATIENT
EDUCATION No treatment plan is complete without addressing issues of
individual and/or group patient education as a means of prolonging the
beneficial effects of treatment, as well as facilitating self-management of
symptoms and injury prevention. The
patient should be encouraged to take an active role in the establishment of
functional outcome goals. They should be
educated on their specific injury, assessment findings, and plan of treatment. Instruction on proper body mechanics and
posture, positions to avoid, self-care for exacerbation of symptoms, and home
exercise should also be addressed.
Time to produce
effect: Varies with individual patient
Frequency: Should occur at every visit
6.9 RESTRICTION
OF ACTIVITIES Continuation of normal daily activities is the recommendation
for acute and chronic pain without neurologic symptoms. There is good evidence against the use of bed
rest in cases without neurologic symptoms.
Bed rest may lead to de-conditioning and impair rehabilitation. Complete work cessation should be avoided, if
possible, since it often further aggravates the pain presentation. Modified return-to-work is almost always more
efficacious and rarely contraindicated in the vast majority of injured workers
with Carpal Tunnel Syndrome
Medication use in the treatment of Carpal
Tunnel Syndrome is appropriate for controlling acute and chronic pain and
inflammation. Use of medications will
vary widely due to the spectrum of injuries from simple strains to
post-surgical healing. All drugs should
be used according to patient needs. A thorough medication history, including
use of alternative and over the counter medications, should be performed at the
time of the initial visit and updated periodically.
6.10 RETURN
TO WORK Early return-to-work should be a prime goal in treating Carpal
Tunnel Syndrome given the poor prognosis for the injured employee who is out of
work for more than six months. The employee and employer should be educated in
the benefits of early return-to-work. When attempting to return an employee
with CTS to the workplace, clear, objective physical restrictions that apply to
both work and non-work related activities should be specified by the provider.
Good communication between the provider, employee, and employer is essential.
Return-to-work is any work or duty that the
employee can safely perform, which may not be the worker's regular job
activities. Due to the large variety of jobs and the spectrum of severity of
CTS, it is not possible for the Division to make specific return-to-work
guidelines, but the following general approach is recommended:
6.10.1 Establishment
of Return-To-Work: Ascertainment of return-to-work status is part of the
medical treatment and rehabilitation plan, and should be addressed at every
visit. Limitations in ADLs should also be reviewed at every encounter, and help
to provide the basis for work restrictions provided they are consistent with
objective findings. The Division recognizes that employers vary in their
ability to accommodate restricted duty, but encourages employers to be active
participants and advocates for early return-to-work. In most cases, the patient can be returned to
work in some capacity, either at a modified job or alternate position, immediately
unless there are extenuating circumstances, which should be thoroughly
documented and communicated to the employer. Return-to-work status should be
periodically reevaluated, at intervals generally not to exceed three weeks, and
should show steady progression towards full activities and full duty.
6.10.2 Establishment
of Activity Level Restrictions: It is the responsibility of the physician/
provider to provide both the employee and employer clear, concise, and specific
restrictions that apply to both work and non-work related activities. The
employer is responsible to determine whether modified duty can be provided
within the medically determined restrictions.
6.10.3 Compliance
with Activity Level Restrictions: The employee's compliance with the activity
level restrictions is an important part of the treatment plan and should be
reviewed at each visit. In some cases, a
job site analysis, a functional capacity evaluation, or other special testing
may be required to facilitate return-to-work and document compliance. Refer to
the “Job Site Alteration” and “Work Tolerance Screening” sections.
6.11 THERAPY-PASSIVE
Passive therapy includes those treatment modalities that do not require energy
expenditure on the part of the patient.
They are principally effective during the early phases of treatment and
are directed at controlling symptoms such as pain, inflammation and swelling
and to improve the rate of healing soft tissue injuries. They should be used in
adjunct with active therapies. They may be used intermittently as a therapist
deems appropriate or regularly if there are specific goals with objectively
measured functional improvements during treatment. Diathermies have not been shown to be
beneficial to patients with CTS and may interfere with nerve conduction.
6.11.1 Manual
Therapy Techniques: are passive interventions in which the providers use
his or her hands to administer skilled movements designed to modulate pain;
increase joint range of motion; reduce/eliminate soft tissue swelling,
inflammation, or restriction; induce relaxation; and improve contractile and
non-contractile tissue extensibility. These techniques are applied only after a
thorough examination is performed to identify those for whom manual therapy
would be contraindicated or for whom manual therapy must be applied with
caution.
6.11.1.1 Mobilization
(Soft Tissue)
Mobilization of soft tissue is the skilled
application of manual techniques designed to normalize movement patterns
through the reduction of soft tissue pain and restrictions.
Indications include muscle spasm around a
joint, trigger points, adhesions, and neural
compression.
Nerve Gliding: consist of a series of flexion and
extension movements of the hand, wrist,
elbow, shoulder, and neck that produce
tension and longitudinal movement along the
length of the median and other nerves of the
upper extremity. These exercises are based
on
the principle that the tissues of the peripheral nervous system are designed for
movement, and that tension and glide
(excursion) of nerves may have an effect on
neurophysiology through alterations in
vascular and axoplasmic flow. Biomechanical
principles have been more thoroughly studied
than clinical outcomes. Nerve gliding
performed on a patient by the clinician
should be reinforced by patient
performance of
similar techniques as part of a home
exercise program at least twice per day.
Time to produce
effect: 4 to 6 treatments
Frequency: 2 to 3 times per week
Maximum duration: 30 visits (CPT codes 97124 and 97140 can not
exceed 30 visits in combination).
6.11.1.2 Massage:
Manual or Mechanical - Massage is manipulation of soft tissue with broad
ranging relaxation and circulatory benefits.
This may include stimulation of acupuncture points and acupuncture
channels (acupressure), application of suction cups and techniques that include
pressing, lifting, rubbing, pinching of soft tissues by or with the
practitioner’s hands. Indications
include edema, muscle spasm, adhesions, the need to improve peripheral
circulation and range of motion, or to increase muscle relaxation and
flexibility prior to exercise.
Time to produce
effect: Immediate.
Frequency: 1 to 3 times per week
Maximum
duration: 12 visits
6.11.2 Ultrasound:
There is some evidence that ultrasound may be effective in symptom relief
and in improving nerve conduction in mild to moderate cases of CTS. No studies
have demonstrated long-term functional benefit. It may be used in conjunction
with an active therapy program for non-surgical patients who do not improve
with splinting and activity modification. It is not known if there are any
long-term deleterious neurological effects from ultrasound.
6.11.3 Microcurrent
TENS and LASER: There is some evidence that concurrent application of
microamperage TENS applied to distinct acupuncture points and low-level laser
treatment may be useful in treatment of mild to moderate CTS. This treatment
may be useful for patients not responding to initial conservative treatment or
who wish to avoid surgery. Patient selection criteria should include absence of
denervation on EMG and motor latencies not exceeding 7 ms. The effects of
microamperage TENS and low-level laser have not been differentiated; there is
no evidence to suggest whether only one component is effective or the
combination of both is required.
Time to produce
effect: 1 week
Frequency: 3 sessions per week
Maximum
duration: 4 weeks
Other Passive
Therapy: For associated myofascial symptoms, please refer to the Cumulative
Trauma Disorder guideline.
6.12 THERAPY-ACTIVE
Active therapies are based on the philosophy that therapeutic exercises and/or
activities are beneficial for restoring flexibility, strength, endurance,
function, range of motion, and alleviating discomfort. Active therapy requires an internal effort by
the individual to complete a specific exercise or task, and thus assists in
developing skills promoting independence to allow self-care to continue after
discharge. This form of therapy requires supervision from a therapist or
medical provider such as verbal, visual, and/or tactile instructions(s). At times a provider may help stabilize the
patient or guide the movement pattern, but the energy required to complete the
task is predominately executed by the patient.
Patients should be instructed to continue
active therapies at home as an extension of the treatment process in order to
maintain improvement levels. Home
exercise can include exercise with or without mechanical assistance or
resistance and functional activities with assistance devices.
Interventions are selected based on the
complexity of the presenting dysfunction with ongoing examination, evaluation
and modification of the plan of care as improvement or lack thereof occurs.
Change and/or discontinuation of an intervention should occur if there is
attainment of expected goals/ outcome, lack of progress, lack of tolerance
and/or lack of motivation. Passive
interventions/ modalities may only be used as adjuncts to the active program.
6.12.1 Activities
of Daily Living: Supervised instruction, active-assisted training, and/or
adaptation of activities or equipment to improve a person’s capacity in normal
daily living activities such as self-care, work re-integration training,
homemaking, and driving.
Time to produce
effect: 4 to 5 treatments
Maximum of 10
sessions
6.12.2 Functional
Activities: are the use of therapeutic activity to enhance mobility, body
mechanics, employability, coordination, and sensory motor integration.
Time to produce
effect: 4 to 5 treatments
Frequency: 3 to 5 times per week
• Maximum duration: 24 visits Total number of visit 97110 and
97530 should not exceed 36 visits without pre-authorization
6.12.3 Neuromuscular
Re-education: is the skilled application of exercise with manual,
mechanical, or electrical facilitation to enhance strength, movement patterns,
neuromuscular response, proprioception, kinesthetic sense, coordination
education of movement, balance, and posture. Indications include the need to
promote neuromuscular responses through carefully timed proprioceptive stimuli,
to elicit and improve motor activity in patterns similar to normal
neurologically developed sequences, and improve neuromotor response with
independent control.
Time to produce
effect: 2 to 6 treatments
Frequency: 3-5 times per week
Maximum
duration: 24 visits
6.12.4 Proper
Work Techniques: Please refer to the “Job Site Evaluation” and “Job Site
Alteration” sections of these guidelines.
6.12.5 Therapeutic
Exercise: with or without mechanical assistance or resistance may include
isoinertial, isotonic, isometric and isokinetic types of exercises. Indications include the need for
cardiovascular fitness, reduced edema, improved muscle strength, improved
connective tissue strength and integrity, increased bone density, promotion of
circulation to enhance soft tissue healing, improvement of muscle recruitment,
increased range of motion, and are used to promote normal movement
patterns. Can also include
complementary/alternative exercise movement therapy.
Time to produce
effect: 2 to 6 treatments
Frequency: 3 to 5 times per week
• Maximum duration: 36 visits Total number of visit 97110 and
97530 should not exceed 36 visits without pre-authorization
7.1 SURGICAL
DECOMPRESSION is well-established, generally accepted, and widely used and
includes open and endoscopic techniques. There is good evidence that surgery is
more effective than splinting in producing long-term symptom relief and
normalization of median nerve conduction velocity.
7.1.1 Endoscopic
Techniques: have had a higher incidence of serious complications (up to 5%)
compared to open techniques (less than 1%). The most commonly seen serious
complications are incomplete transection of the transverse carpal ligament and
inadvertent nerve or vessel injuries.
The incidence of complications may be lower for surgeons who have
extensive experience and familiarity with certain endoscopic techniques. Choice
of technique should be left to the discretion of the surgeon.
7.1.2 Indications
for Surgery: include positive history, abnormal electrodiagnostic studies,
and/or failure of conservative management. Job modification should be
considered prior to surgery. Please refer to the “Job Site Alteration” section
for additional information on job modification.
7.1.3 Surgery
as an Initial Therapy: Surgery should be considered as an initial therapy
in situations where:
7.1.3.1 Median
nerve trauma has occurred; “acute carpal tunnel syndrome”, or
7.1.3.2 Electrodiagnostic
evidence of moderate to severe neuropathy. EMG findings showing evidence of
acute or chronic motor denervation suggest the possibility that irreversible
damage may be occurring.
7.1.4 Surgery
When Electrodiagnostic Testing is Normal: Surgery may be considered in
cases where electrodiagnostic testing is normal. An opinion from a hand surgeon
mayshouldmay be considered. The following criteria should be considered in
deciding whether to proceed with surgery:
7.1.4.1 The
patient experiences significant temporary relief following steroid injection
into the carpal tunnel; or
7.1.4.2 The
patient has failed 3-6 months of conservative treatment including work site
change, if such changes are available; and
7.1.4.3 The
patient's signs and symptoms are specific for carpal tunnel syndrome
7.1.5 Suggested
parameters for return-to-work are:
Time Frame Activity Level
2 Days Return to Work with Restrictions on utilizing the affected
extremity 2-3 Weeks Sedentary and
non-repetitive work 4-6 Weeks Case-by-case basis 6-12 Weeks Heavy Labor,
forceful and repetitive
Note: All return-to-work decisions are based upon
clinical outcome.
7.2 NEUROLYSIS
has not been proven advantageous for carpal tunnel syndrome. Internal neurolysis should never be done.
Very few indications exist for external neurolysis.
7.3 TENOSYNOVECTOMY
has not proven to be of benefit in primary carpal tunnel syndrome but
occasionally can be beneficial in certain patients with co-existing or systemic
disorders.
7.4 CONSIDERATIONS
FOR REPEAT SURGERY The single most important factor in predicting
symptomatic improvement following carpal tunnel release is the severity of
preoperative neuropathy. Patients with moderate electrodiagnostic abnormalities
have better results than those with either very severe or no abnormalities.
Incomplete cutting of the transverse carpal ligament or iatrogenic injury to
the median nerve are rare.
If median nerve symptoms do not improve following
initial surgery or symptoms improve initially and then recur, but are
unresponsive to non-operative therapy (see Section.F, Therapeutic Procedures,
Non-Operative) consider the following:
7.4.1 Recurrent
synovitis;
7.4.2 Repetitive
work activities may be causing “dynamic” CTS;
7.4.3 Scarring;
7.4.4 Work-up
of systemic diseases A second opinion by a hand surgeon and new
electodiagnosticelectrodiagnostic studies required if repeat surgery is
contemplated. The decision to undertake repeat surgery must
factor
in all of the above possibilities. Results of surgery for recurrent carpal
tunnel syndrome vary widely depending on the etiology of recurrent symptoms.
7.5 POST-OPERATIVE
TREATMENT Considerations for post-operative therapy are:
7.5.1 Immobilization:
There is some evidence showing that immediate mobilization of the wrist
following surgery is associated with less scar pain and faster return to work.
Final decisions regarding the need for splinting post-operatively should be
left to the discretion of the treating physician based upon his/her
understanding of the surgical technique used and the specific conditions of the
patient.
7.5.2 Home
Program: It is generally accepted that all patients should receive a home
therapy protocol involving stretching, ROM, scar care, and resistive exercises.
Patients should be encouraged to use the hand as much as possible for daily
activities, allowing pain to guide their activities.
7.5.3 Supervised
Therapy Program: may be helpful in patients who do not show functional
improvements post-operatively, in patients with heavy or repetitive job
activities and certain high-risk patients. The therapy program may include some
of the generally accepted elements of soft tissue healing and return to
function:
7.5.3.1 Soft
tissue healing/remodeling: May be used after the incision has healed. It may
include all of the following: evaluation, whirlpool, electrical stimulation,
soft tissue mobilization, scar desensitivation, heat/cold application,
splinting or edema control may be used as indicated. Following wound healing,
ultrasound and iontophoresis with Sodium Chloride (NaCl) may be considered for
soft tissue remodeling. Diathermy is a non-acceptable adjunct.
7.5.3.2 Return
to function: Range of motion and stretching exercises, strengthening, activity
of daily living adaptations, joint protection instruction, posture/body
mechanics education; worksite modifications may be indicated.
Time to produce
effect: 2-4 weeks
Frequency: 2-5 times/week
Maximum duration:
36 visits
PART B CHRONIC PAIN TREATMENT GUIDELINES
Pursuant to 19 Del.C. §2322C, health
care practice guidelines have been adopted and recommended by the Health Care
Advisory Panel to guide utilization of health care treatments in workers'
compensation including, but not limited to, care provided for the treatment of
employees by or under the supervision of a licensed health care provider,
prescription drug utilization, inpatient hospitalization and length of stay,
diagnostic testing, physical therapy, chiropractic care and palliative care.
The health care practice guidelines apply to all treatments provided after the
effective date of the regulation adopted by the Department of Labor, May 23,
2008, and regardless of the date of injury. The guidelines are, to the extent
permitted by the most current medical science or applicable science, based on
well-documented scientific research concerning efficacious treatment for
injuries and occupational disease. To the extent that well-documented
scientific research regarding the above is not available at the time of
adoption of the guidelines, or is not available at the time of any revision to
the guidelines, the guidelines have been and will be based upon the best
available information concerning national consensus regarding best health care
practices in the relevant health care community.
The
guidelines, to the extent practical and consistent with the Act, address
treatment of those physical conditions which occur with the greatest frequency,
or which require the most expensive treatments, for work-related injuries based
upon currently available Delaware
data.
Services
rendered by any health care provider certified pursuant to 19 Del.C.
§2322D(a) to provide treatment or services for injured employees shall be
presumed, in the absence of contrary evidence, to be reasonable and necessary
if such treatment and/or services conform to the most current version of the
Delaware health care practice guidelines.
Services
rendered outside the Guidelines and/or variation in treatment recommendations
from the Guidelines may represent acceptable medical care, be considered
reasonable and necessary treatment and, therefore, determined to be
compensable, absent evidence to the contrary, and may be payable in accordance
with the Fee Schedule and Statute, accordingly.
Services
provided by any health care provider that is not certified pursuant to 19 Del.C.
§2322D(a) shall not be presumed reasonable and necessary unless such services
are pre-authorized by the employer or insurance carrier, subject to the
exception set forth in 19 Del.C. §2322D(b).
Treatment
of conditions unrelated to the injuries sustained in an industrial accident may
be denied as unauthorized if the treatment is directed toward the
non-industrial condition, unless the treatment of the unrelated injury is
rendered necessary as a result of the industrial accident.
The
Health Care Advisory Panel and Department of Labor recognized that acceptable
medical practice may include deviations from these Guidelines, as individual
cases dictate. Therefore, these Guidelines are not relevant as evidence of a
provider's legal standard of professional care.
In accordance with the requirements of the
Act, the development of the health care guidelines has been directed by a
predominantly medical or other health professional panel, with recommendations
then made to the Health Care Advisory Panel.
The
principles summarized in this section are key to the intended implementation of
all Office of Workers’ Compensation guidelines and critical to the reader’s
application of the guidelines in this document.
2.1 TREATMENT
PARAMETER DURATION Time frames for specific interventions commence once
treatments have been initiated, not on the date of injury. Obviously, duration
will be impacted by patient compliance, as well as availability of services.
Clinical judgment may substantiate the need to accelerate or decelerate the
time frames discussed in this document.
2.2 ACTIVE
INTERVENTIONS emphasizing patient responsibility, such as therapeutic
exercise and/or functional treatment, are generally emphasized over passive
modalities, especially as treatment progresses. Generally, passive
interventions are viewed as a means to facilitate progress in an active
rehabilitation program with concomitant attainment of objective functional gains.
2.3 ACTIVE
THERAPEUTIC EXERCISE PROGRAM Exercise program goals should incorporate
patient strength, endurance, flexibility, coordination, and education. This
includes functional application in vocational or community settings.
2.4 POSITIVE
PATIENT RESPONSE Positive results are defined primarily as functional gains
that can be objectively measured. Objective functional gains include, but are
not limited to, positional tolerances, range of motion (ROM), strength, endurance
activities of daily living cognition, psychological behavior, and
efficiency/velocity measures that can be quantified. Subjective reports of pain
and function should be considered and given relative weight when the pain has
anatomic and physiologic correlation.
2.5 RE-EVALUATION
OF TREATMENT EVERY 3 TO 4 WEEKS With respect to Therapy (Active or
Passive), if a given treatment or modality is not producing positive results
within 3 to 4 weeks, the treatment should be either modified or discontinued.
Reconsideration of diagnosis should also occur in the event of poor response to
a seemingly rational intervention.
2.6 SURGICAL
INTERVENTIONS Surgery should be contemplated within the context of expected
functional outcome and not purely for the purpose of pain relief. The concept
of “cure” with respect to surgical treatment by itself is generally a misnomer.
All operative interventions must be based upon positive correlation of clinical
findings, clinical course, and diagnostic tests. A comprehensive assimilation
of these factors must lead to a specific diagnosis with identification of
pathologic conditions.
2.7 RETURN-TO-WORK
is therapeutic, assuming the work is not likely to aggravate the basic
problem or increase long-term pain. The practitioner must provide specific
written physical limitations and the patient should never be released to
“sedentary” or “light duty.” The following physical limitations should be
considered and modified as recommended: lifting, pushing, pulling, crouching,
walking, using stairs, overhead work, bending at the waist, awkward and/or
sustained postures, tolerance for sitting or standing, hot and cold
environments, data entry and other repetitive motion tasks, sustained grip,
tool usage and vibration factors. Even if there is residual chronic pain,
return-to-work is not necessarily contraindicated.
The practitioner should understand all of
the physical demands of the patient’s job position before returning the patient
to full duty and should request clarification of the patient’s job duties.
2.8 DELAYED
RECOVERY Strongly consider a psychological evaluation, if not previously
provided, as well as initiating interdisciplinary rehabilitation treatment and
vocational goal setting, for those patients who are failing to make expected
progress 6 to 12 weeks after an injury. The Office of Workers Compensation recognizes that 3 to 10%
of all industrially injured patients will not recover within the time lines
outlined in this document despite optimal care. Such individuals may require
treatments beyond the limits discussed within this document, but such treatment
will require clear documentation by the authorized treating practitioner
focusing on objective functional gains afforded by further treatment and impact
upon prognosis.
2.9 GUIDELINE
RECOMMENDATIONS AND INCLUSION OF MEDICAL EVIDENCE recommendations are based
on available evidence and/or consensus recommendations of the standard of care
within Delaware.
Those procedures considered inappropriate, unreasonable, or unnecessary are
designated in the guideline as being “not recommended.”
2.10 TREATMENT
OF PRE-EXISTING CONDITIONS that preexisted the work injury/disease will
need to be managed under two circumstances: (a) A pre-existing condition
exacerbated by a work injury/ disease should be treated until the patient has
returned to their prior level of functioning; and
(b) A pre-existing condition not directly
caused by a work injury/disease but which may prevent recovery from that injury
should be treated until its negative impact has been controlled. The focus of
treatment should remain on the work injury/disease.
The International Association for the Study
of Pain (IASP) defines pain as “an unpleasant sensory and emotional experience
with actual or potential tissue damage.” Pain is a complex experience embracing
physical, mental, social, and behavioral processes that often compromises the
quality of life of many individuals. Pain is an unpleasant subjective
perception usually in the context of tissue damage. Pain is subjective and
cannot be measured or indicated objectively. Pain evokes negative emotional
reactions such as fear, anxiety, anger, and depression. People usually regard
pain as an indicator of physical harm, despite the fact that pain can exist
without tissue damage and tissue damage can exist without pain. Many people
report pain in the absence of tissue damage or any likely pathophysiologic
cause. There is no way to distinguish their experience from that due to actual
tissue damage. If they regard their experience as pain and they report it the
same way as pain caused by tissue damage, it should be accepted as pain. Pain
can generally be classified as:
Nociceptive which includes pain from visceral origins or damage to other
tissues. Myofascial pain is a nociceptive type of pain characterized by
myofascial trigger points limited to a specific muscle or muscles. Neuropathic
including that originating from brain, peripheral nerves or both; and
Psychogenic that originates in mood, characterological, social, or
psychophysiological processes.
Recent advances in the neurosciences reveal additional mechanisms
involved in chronic pain. In the past, pain was seen as a sensation arising
from the stimulation of pain receptors by damaged tissue, initiating a sequence
of nerve signals ending in the brain and there recognized as pain. A
consequence of this model was that ongoing pain following resolution of tissue
damage was seen as less physiological and more psychological than acute pain
with identifiable tissue injury. Current research indicates that chronic pain
involves additional mechanisms that cause: 1) neural remodeling at the level of
the spinal cord and higher levels of the central nervous system; 2) changes in
membrane responsiveness and connectivity leading to activation of larger pain
pathways; and 3) recruitment of distinct neurotransmitters. Changes in gene
function and expression may occur, with lasting functional consequences. These
physiologic functional changes cause chronic pain to be experienced in body
regions beyond the original injury and to be exacerbated by little or no
stimulation. The chronic pain experience clearly represents both psychologic
and complex physiologic mechanisms, many of which are just beginning to be
understood. Chronic Pain is defined as "pain that persists for at least 30
days beyond the usual course of an acute disease or a reasonable time for an
injury to heal or that is associated with a chronic pathological process that
causes continuous pain (e.g., reflex sympathetic dystrophy)." The very
definition of chronic pain describes a delay or outright failure to relieve
pain associated with some specific illness or accident. Delayed recovery should
prompt a clinical review of the case and a psychological evaluation by the
health care provider. Referral to a recognized pain specialist for further
evaluation is recommended. Consideration may be given to new diagnostic testing
or a change in treatment plan.
Chronic pain is a phenomenon not specifically relegated to anatomical or
physiologic parameters. The prevailing biomedical model (which focuses on
identified disease pathology as the sole cause of pain) cannot capture all of
the important variables in pain behavior. While diagnostic labels may pinpoint
contributory physical and/or psychological factors and lead to specific
treatment interventions that are helpful, a large number of patients defy
precise taxonomic classification. Furthermore, such diagnostic labeling often
overlooks important social contributions to the chronic pain experience.
Failure to address these operational parameters of the chronic pain experience
may lead to incomplete or faulty treatment plans. The term "pain
disorder" is perhaps the most useful term in the medical literature today,
in that it captures the multi-factorial nature of the chronic pain experience.
It is recognized that some health care practitioners, by virtue of their
experience, additional training, and/or accreditation by pain specialty
organizations, have much greater expertise in the area of chronic pain
evaluation and treatment than others. Referrals for the treatment of chronic
pain should be to such recognized specialists. Chronic pain treatment plans
should be monitored and coordinated by pain medicine physicians with such specialty
training, in conjunction with other health care specialists.
Most acute and some chronic pain problems are adequately addressed in
other Office of Workers' Compensation treatment guidelines, and are generally beyond the scope of
these guidelines. However, because chronic pain is more often than not
multi-factorial, involving more than one pathophysiologic or mental disorder,
some overlap with other guidelines is inevitable. These guidelines are meant to
apply to any patient who fits the operational definition of chronic pain
discussed at the beginning of this section.
Aftersensation
Refers to the abnormal persistence of a sensory perception, provoked by a
stimulus even though the stimulus has ceased. Allodynia Pain due to a non-noxious
stimulus that does not normally provoke pain.
Dynamic Mechanical Allodynia – Obtained by moving the stimulus such as a brush or cotton tip
across the abnormal hypersensitive area.
Mechanical Allodynia – Refers to the abnormal perception of pain from
usually non-painful
mechanical stimulation.
Static Mechanical Allodynia – Refers to pain obtained by applying a
single stimulus such as light
pressure to a defined area.
Thermal Allodynia – Refers to the abnormal sensation of pain from usually
non-painful thermal
stimulation such as cold or warmth.
Analgesia Absence of
pain in response to stimulation that would normally be painful.
Biopsychosocial A
term that reflects the multiple facets of any clinical situation; namely, the
biological, psychological, and social situation of the patient.
Central Pain Pain initiated or caused by a primary lesion or dysfunction
in the central nervous system.
Central Sensitization The experience of pain evoked by the excitation of
non-nociceptive neurons or
of
nerve fibers that normally relay non-painful sensations to the spinal cord.
This results when non-
nociceptive
afferent neurons act on a sensitized central nervous system (CNS).
Dysesthesia An abnormal sensation described by the patient as
unpleasant. As with paresthesia,
dysesthesia may be spontaneous or evoked by maneuvers on physical examination.
Hyperalgesia Refers
to an exaggerated pain response from a usually painful stimulation.
Hyperesthesia (Positive Sensory Phenomena) Includes allodynia,
hyperalgesia, and hyperpathia.
Elicited
by light touch, pin prick, cold, warm, vibration, joint position sensation or
two-point
discrimination, which is perceived as increased or more.
Hyperpathia Refers to an abnormally painful and exaggerated reaction to
stimulus, especially to a
repetitive
stimulus.
Hypoalgesia Diminished pain perception in response to a normally painful
stimulus.
Hypoesthesia (Negative Sensory Phenomena) Refers to a stimulus such as light touch, pin
prick,
cold,
point position sensation, two-point discrimination, or sensory neglect which is
perceived as
decreased.
Malingering Intentional
feigning of illness or disability in order to escape work or gain compensation.
Myofascial Pain A regional pain characterized by tender points in taut
bands of muscle that produce
pain in a characteristic reference zone.
Myofascial Trigger
Point A physical sign in a muscle which includes a)
exquisite tenderness in a taut
muscle band; and b) referred pain elicited by mechanical stimulation of the
trigger point. The following
findings may be associated with myofascial trigger points: 1) Local twitch or
contraction of the taut
band when the trigger point is mechanically stimulated; 2) Reproduction of the
patient’s spontaneous
pain pattern when the trigger point is mechanically stimulated; 3) Weakness
without muscle atrophy; 4)
Restricted range of motion of the affected muscle; and 5) Autonomic dysfunction
associated with the
trigger point such as changes in skin or limb temperature.
Neuralgia Pain in
the distribution of a nerve or nerves.
Neuritis Inflammation of a nerve or nerves.
Neurogenic Pain Pain initiated or caused by a primary lesion,
dysfunction, or transitory perturbation in
the
peripheral or central nervous system.
Neuropathic Pain Pain due to an injured or dysfunctional central or
peripheral nervous system.
Neuropathy A disturbance of function or pathological change in a nerve:
in one nerve,
mononeuropathy;
in several nerves, mononeuropathy multiplex; if diffuse and bilateral,
polyneuropathy.
Nociceptor A
receptor preferentially sensitive to a noxious stimulus or to a stimulus which
would
become
noxious if prolonged.
Pain Behavior The non-verbal actions (such as grimacing, groaning,
limping, using visible pain
relieving or support devices and requisition of pain medications, among others)
that are outward
manifestations of pain, and through which a person may communicate that pain is
being experienced.
Pain Threshold The
smallest stimulus perceived by a subject as painful.
Paresthesia An abnormal sensation that is not described as pain. It can
be either a spontaneous
sensation
(such as pins and needles) or a sensation evoked from non-painful or painful
stimulation,
such as light touch, thermal, or pinprick stimulus on physical examination.
Peripheral Neurogenic Pain Pain initiated or caused by a primary lesion
or dysfunction or transitory
perturbation
in the peripheral nervous system.
Peripheral Neuropathic Pain Pain initiated or caused by a primary lesion or dysfunction in the
peripheral nervous system.
Summation Refers to abnormally painful sensation to a repeated stimulus
although the actual
stimulus
remains constant. The patient describes the pain as growing and growing as the
same
intensity
stimulus continues.
Sympathetically Maintained Pain (SMP) A pain that is maintained by
sympathetic efferent
innervations or by circulating catecholamines.
Tender Points Tenderness on palpation at a tendon
insertion, muscle belly or over bone. Palpation
should be done with the thumb or forefinger, applying pressure approximately
equal to a force of 4
kilograms (blanching of the entire nail bed).
The
Department recommends the following diagnostic procedures be considered, at
least initially, the responsibility of the workers’ compensation carrier to
ensure that an accurate diagnosis and treatment plan can be established.
Standard procedures that should be utilized when initially diagnosing a
work-related chronic pain complaint are listed below.
5.1 HISTORY
AND PHYSICAL EXAMINATION (HX & PE)
5.1.1 Medical
History: As in other fields of medicine, a thorough patient history is an
important part of the evaluation of chronic pain. In taking such a history,
factors influencing a patient’s current status can be made clear and taken into
account when planning diagnostic evaluation and treatment. One efficient manner
in which to obtain historical information is by using a questionnaire. The
questionnaire may be sent to the patient prior to the initial visit or
administered at the time of the office visit.
5.1.2 Pain
History: Characterization of the patient’s pain and of the patient’s response
to pain is one of the key elements in treatment.
5.1.3 Medical
Management History
5.1.4 Substance
Use/Abuse
5.1.5 Other
Factors Affecting Treatment Outcome
5.1.6 Physical
Examination
5.2 DIAGNOSTIC
STUDIES Imaging of the spine and/or extremities is a generally accepted,
well-established, and widely used diagnostic procedure when specific
indications, based on history and physical examination, are present.
5.2.1 Radiographic
Imaging, MRI, CT, bone scan, radiography, and other special imaging studies may
provide useful information for many musculoskeletal disorders causing chronic
pain.
5.2.2 Electrodiagnostic
studies may be useful in the evaluation of patients with suspected myopathic or
neuropathic disease and may include Nerve Conduction Studies (NCS), Standard
Needle Electromyography, or Somatosensory Evoked Potential (SSEP). The
evaluation of electrical studies is difficult and should be relegated to
specialists who are well trained in the use of this diagnostic procedure.
5.2.3 Special
Testing Procedures may be considered when attempting to confirm the current
diagnosis or reveal alternative diagnosis. In doing so, other special tests may
be performed at the discretion of the physician.
5.3 LABORATORY
TESTING is generally accepted well-established and widely used procedures
and can provide useful diagnostic and monitoring information. They may be used
when there is suspicion of systemic illness, infection, neoplasia, or
underlying rheumatologic disorder, connective tissue disorder, or based on
history and/or physical examination. Tests include, but are not limited to:
5.3.1 Complete
Blood Count (CBC) with differential can detect infection, blood dyscrasias, and
medication side effects;
5.3.2 Erythrocyte
sedimentation rate, rheumatoid factor, antinuclear antigen (ANA), human
leukocyte antigen (HLA), and C-reactive protein can be used to detect evidence
of a rheumatologic, infection, or connective tissue disorder;
5.3.3 Thyroid,
glucose and other tests to detect endocrine disorders;
5.3.4 Serum
calcium, phosphorous, uric acid, alkaline phosphatase, and acid phosphatase can
detect metabolic bone disease;
5.3.5 Urinalysis
to detect bacteria (usually with culture and sensitivity), calcium, phosphorus,
hydroxyproline, or hematuria;
5.3.6 Liver
and kidney function may be performed for baseline testing and monitoring of
medications; and
5.3.7 Toxicology
Screen and/or Blood Alcohol Level if suspected drug or alcohol abuse.
5.4 INJECTIONS–DIAGNOSTIC
5.4.1 Spinal
Diagnostic Injections: Description — generally accepted, well-established
procedures. These injections may be useful for localizing the source of pain,
and may have added therapeutic value when combined with injection of
therapeutic medication(s). Selection of patients, choice of procedure, and
localization
of
the level for injection should be determined by clinical information indicating
strong suspicion for
pathologic condition(s) and the source of pain symptoms.
The interpretation of the test results are primarily based on functional
change, symptom report,
and pain response (via a recognized pain
scale before and at an appropriate time after the injection). The diagnostic
significance of the test result should be evaluated in conjunction with clinical
information and the results of other diagnostic procedures. Injections with
local anesthetics of differing duration may be used to support a diagnosis. In
some cases, injections at multiple levels may be required to accurately
diagnose conditions. Regarding
diagnostic injections, it is obligatory that sufficient data be accumulated by
the examiner performing this procedure such that the diagnostic value of the
procedure is evident to other reviewers. A log must be recorded as part of the
medical record which documents response, if any, on an hourly basis for, at a
minimum, the expected duration of the local anesthetic phase of the procedure.
Responses should be identified as to specific body part (e.g., low back, neck,
leg, or arm pain).
Special Requirements for Diagnostic
Injections - Since multi-planar, fluoroscopy during procedures is required to
document technique and needle placement, an experienced physician should
perform the procedure. Permanent images are required to verify needle placement
for all spinal procedures. The subspecialty disciplines of the physicians
performing injections may be varied, including, but not limited to:
anesthesiology, radiology, surgery, or physiatry. The practitioner who performs
spinal injections should document hands-on training through workshops of the
type offered by organizations such as the International Spine Intervention
Society (ISIS) and/or completed fellowship training with interventional
training. Practitioners performing
spinal injections for low back and cervical pain must also be knowledgeable in
radiation safety.
Specific Diagnostic Injections - In general, relief should last for at
least the duration of the local anesthetic used and/or should significantly
relieve pain and result in functional improvement. The following injections are
used primarily for diagnosis:
5.4.1.1
Medial Branch Blocks: Medial Branch Blocks are primarily diagnostic injections,
used to determine whether a patient is a candidate for radiofrequency medial
branch neurotomy (also known as facet rhizotomy). To be a positive diagnostic
block, the patient should report a reduction of pain of 50% or greater relief
from baseline for the length of time appropriate for the local anesthetic used.
It is suggested that this be reported on a form. A separate block on a
different date should be performed to confirm the level of involvement.
Frequency and Maximum Duration: May be repeated once for comparative blocks.
Limited to 4 levels.
5.4.1.2 Transforaminal
Injections are useful in identifying spinal pathology. When performed for
diagnosis, small amounts of local anesthetic up to a total volume of 1.0 cc
should be used to determine the level of nerve root irritation. A positive
diagnostic block should result in a 50% reduction in nerve-root generated pain
appropriate for the anesthetic used as measured by accepted pain scales (such
as a VAS).
Frequency and Maximum Duration: Once per suspected level. Limited to
three levels, may be repeated for confirmation.
5.4.1.3 Zygapophyseal
(facet) blocks: Facet blocks are generally. They may be used diagnostically to
direct functional rehabilitation programs. A positive diagnostic block should
result in a positive diagnostic functional benefit and/or a 50% reduction in
pain appropriate for the anesthetic used as measured by accepted pain
scales (such as a Visual Analog Scale). They then may be repeated per
the therapeutic guidelines Frequency and Maximum Duration: Once per suspected
level, limited to three levels, may
be repeated for confirmation.
5.4.1.4 Atlanto-Axial
and Atlanto-Occipital Injections: are generally accepted for diagnosis and
treatment but do not lend themselves to denervation techniques owing to
variable neuroanatomy.
Frequency and Maximum Duration: Once per side
5.4.1.5 Sacroiliac
Joint Injection:
Description - a generally accepted injection of local anesthetic in an
intra-articular fashion
into the sacroiliac joint under fluoroscopic guidance.
Indications - Primarily diagnostic to rule out sacroiliac joint
dysfunction versus other pain
generators. Intra-articular injection can be
of value in diagnosing the pain generator. There
should be at least 50% pain relief.
Frequency and Maximum Duration: 1 may be repeated for confirmation.
Non-operative
therapeutic rehabilitation is applied to patients with chronic and complex
problems of de-conditioning and functional disability. Treatment modalities may
be utilized sequentially or concomitantly depending on chronicity and
complexity of the problem, and treatment plans should always be based on a
diagnosis utilizing appropriate diagnostic procedures.
Before
initiation of any therapeutic procedure, the authorized treating physician,
employer, and insurer must consider these important issues in the care of the
injured worker:
6.1 Patients
undergoing therapeutic procedure(s) should be released or returned to modified
or restricted duty during their rehabilitation at the earliest appropriate
time. Refer to Return-to-Work in this section for detailed information.
6.2 Reassessment
of the patient’s status in terms of functional improvement should be documented
after each treatment. If patients are not responding within the recommended
time periods, alternative treatment interventions, further diagnostic studies
or consultations should be pursued. Continued treatment should be monitored
using objective measures such as:
Return-to-work
or maintaining work status Fewer restrictions at work or performing activities
of daily living. Decrease in usage of medications Measurable functional gains,
such as increased range of motion or documented increase in strength.
6.3 Clinicians
should provide and document education to the patient. No treatment plan is
complete without addressing issues of individual and/or group patient education
as a means of facilitating self-management of symptoms.
6.4 Psychological or psychosocial screening should be performed on
all chronic pain patients. The following procedures are listed in alphabetical
order.
6.4.1 ACUPUNCTURE
is an accepted and widely used procedure for the relief of pain and inflammation. The exact mode of action is only partially understood. Western medicine studies suggest that acupuncture stimulates the nervous system at the level of the brain, promotes deep relaxation, and affects the release of neurotransmitters. Acupuncture is commonly used as an alternative or in addition to traditional Western pharmaceuticals. While it is commonly used when pain medication is reduced or not tolerated, it may be used as an adjunct to physical rehabilitation and/or surgical intervention to hasten the return of functional activity. Acupuncture should be performed by MD, DO, DC with appropriate training; or a licensed acupuncturist.
6.4.1.1 Acupuncture:
is the insertion and removal of filiform needles to stimulate acupoints
(acupuncture points). Needles may be inserted, manipulated, and retained for a
period of time. Acupuncture can be used to reduce pain, reduce inflammation,
increase blood flow, increase range of motion, decrease the side effect of
medication-induced nausea, promote relaxation in an anxious patient, and reduce
muscle spasm.
Indications include joint pain, joint
stiffness, soft tissue pain and inflammation, paresthesia, post-surgical pain
relief, muscle spasm, and scar tissue pain.
6.4.1.2 Acupuncture
with Electrical Stimulation: is the use of electrical current (microamperage
or milli-amperage) on the needles at the acupuncture site. It is used to
increase effectiveness of the needles by continuous stimulation of the
acupoint. Physiological effects (depending on location and settings) can
include endorphin release for pain relief, reduction of inflammation, increased
blood circulation, analgesia through interruption of pain stimulus, and muscle
relaxation.
It is indicated to treat chronic pain
conditions, radiating pain along a nerve pathway, muscle spasm, inflammation,
scar tissue pain, and pain located in multiple sites.
6.4.1.3 Total
Time Frames For Acupuncture and Acupuncture with Electrical Stimulation: Time
frames are not meant to be applied to each of the above sections separately.
The time frames are to be applied to all acupuncture treatments regardless of
the type or combination of therapies being provided.
Time to produce effect: 3 to 6 treatments Frequency: 1 to 3 times per
week Maximum course duration: 14 treatments (one course)
Any of the above acupuncture treatments may
extend longer if objective functional gains can be documented or when
symptomatic benefits facilitate progression in the patient’s treatment program.
An additional course of treatment beyond 14 treatments may be documented with
respect to need and ability to facilitate positive symptomatic or functional
gains. Such care should be re-evaluated and documented with each series of
treatments.
6.4.1.4 Other
Acupuncture Modalities: Acupuncture treatment is based on individual
patient needs and therefore treatment may include a combination of procedures
to enhance treatment effect. Other procedures may include the use of heat, soft
tissue manipulation/ massage, and exercise. Refer to Active Therapy
(Therapeutic Exercise) and Passive Therapy sections (Massage and Superficial
Heat and Cold Therapy) for a description of these adjunctive acupuncture
modalities and time frames.
6.4.2 BIOFEEDBACK
is a generally well-accepted form of behavioral medicine that helps patients
learn self-awareness and self-regulation skills for the purpose of gaining
greater control of their physiology. Stress-related psycho physiological
reactions may arise as a reaction to organic pain and in some cases may cause
pain. Electronic instrumentation is used to monitor the targeted physiology and
then displayed or fed back to the patient visually, auditorially, or tactilely
with
coaching
by a biofeedback specialist. Indications for biofeedback include individuals
who are suffering from musculoskeletal injury where muscle dysfunction or other
physiological indicators of excessive or prolonged stress response affects
and/or delays recovery. Other applications include training to improve self-management
of pain, anxiety, panic, anger or emotional distress, narcotic withdrawal,
insomnia/ sleep disturbance, and other central and autonomic nervous system
imbalances. Biofeedback is often utilized for relaxation training. Mental
health professionals may also utilize it as a component of psychotherapy, where
biofeedback and other behavioral techniques are integrated with
psychotherapeutic interventions. Biofeedback is often used in conjunction with
physical therapy or medical treatment. Recognized types of biofeedback include
the following:
6.4.2.1 Electromyogram
(EMG): Used for self-management of pain and stress reactions involving
muscle tension.
6.4.2.2 Skin
Temperature: Used for self-management of pain and stress reactions,
especially vascular headaches.
6.4.2.3 Respiration
Feedback (RFB): Used for self-management of pain and stress reactions via
breathing control.
6.4.2.4 Respiratory
Sinus Arrhythmia (RSA): Used for self-management of pain and stress
reactions via synchronous control of heart rate and respiration. Respiratory
sinus arrhythmia is a benign phenomena which consists of a small rise in heart
rate during inhalation, and a corresponding decrease during exhalation. This
phenomenon has been observed in meditators and athletes, and is thought to be a
psycho physiological indicator of health.
6.4.2.5 Heart
Rate Variability (HRV): Used for self-management of stress via managing
cardiac reactivity.
6.4.2.6 Electrodermal
Response (EDR): Used for self-management of stress involving palmar
sweating or galvanic skin response.
6.4.2.7 Electroencephalograph
(EEG, QEEG): Used for self-management of various psychological states by
controlling brainwaves.
The goal in biofeedback treatment is normalizing the physiology to the
pre-injury status to
the
extent possible and involves transfer of learned skills to the workplace and
daily life.
Candidates
for biofeedback therapy or training must be motivated to learn and practice
biofeedback
and self-regulation techniques. In the course of biofeedback treatment,
patient
stressors are discussed and self-management strategies are devised. If the
patient
has
not been previously evaluated, a psychological evaluation should be performed
prior
to
beginning biofeedback treatment for chronic pain. The psychological evaluation
may
reveal
cognitive difficulties, belief system conflicts, somatic delusions, secondary
gain
issues,
hypochondriasis, and possible biases in patient self-reports, which can affect
biofeedback.
Home practice of skills is often helpful for mastery and may be facilitated by
the
use of home training tapes.
Psychologists or psychiatrists, who provide
psychophysiological
therapy which integrates biofeedback with psychotherapy, should be
either
Biofeedback Certification Institute of America (BCIA) certified or practicing
within
the
scope of their training. All other providers of Biofeedback for chronic pain
patients
must
be BCIA certified and shall have their biofeedback treatment plan approved by
the
authorized
treating psychologist or psychiatrist. Biofeedback treatment must be done in
conjunction
with the patient’s psychosocial intervention. Biofeedback may also be
provided
by unlicensed health care providers, who follow a set treatment and educational
protocol.
Such treatment may utilize standardized material or relaxation tapes.
Time to produce effect: 3 to 4 sessions
Frequency: 1 to 2 times per week
Optimum duration: 6 to 8 sessions
Maximum duration: 10 to 12 sessions.
Treatment beyond 12 sessions must be documented with respect to need,
expectation,
and ability to facilitate positive symptomatic or functional gains.
6.4.3 COMPLEMENTARY
ALTERNATIVE MEDICINE (CAM) is a term used
to describe a broad range of treatment modalities, a number of which are
generally accepted and supported by some scientific evidence, and others which
still remain outside the generally accepted practice of conventional Western
Medicine. In many of these approaches, there is attention given to the
relationship between physical, emotional, and spiritual well-being. While CAM
may be performed by a myriad of both licensed and non-licensed health
practitioners with training in one or more forms of therapy, credentialed
practitioners should be used when available or applicable.
Although CAM
practices are diverse and too numerous to list, they can be generally
classified into five domains:
6.4.3.1 Alternative
Medical Systems: These are defined as medical practices that have developed
their own systems of theory, diagnosis and treatment and have evolved
independent of and usually prior to conventional Western Medicine. Some
examples are Traditional Chinese Medicine, Ayurvedic Medicine, Homeopathy, and
Naturopathy.
6.4.3.2 Mind-Body
Interventions: These include practices such as hypnosis, meditation,
bioenergetics, and prayer.
6.4.3.3 Biological-based
Practices: These include herbal and dietary therapy as well as the use of
nutritional supplements. To avoid potential drug interactions, supplements
should be used in consultation with the authorized treating physician.
6.4.3.4 Body-Based
Therapy: Included in this category are the practices of Yoga and Rolfing
bodywork.
6.4.3.5 Energy-Based
Practices: Energy-based practices include a wide range of modalities that
support physical as well as spiritual and/or emotional healing. Some of the more well-known energy practices
include Qi Gong, Tai Chi, Healing Touch and Reiki. Practices such as Qi Gong
and Tai Chi are taught to the patient and are based on exercises the patient
can practice independently at home. Other energy-based practices such as
Healing Touch and Reiki involve a practitioner/patient relationship.
6.4.3.6 Methods used to evaluate chronic pain
patients for participation in CAM will differ
with various approaches and with the training and experience of individual
practitioners. A patient may be referred for CAM therapy when the patient’s
cultural background, religious beliefs, or personal concepts of health suggest
that an unconventional medical approach might assist in the patient’s recovery
or when the physician’s experience and clinical judgment support a CAM approach. The patient must demonstrate a high degree
of motivation to return to work and improve their functional activity level
while participating in therapy. Other more traditional conservative treatments
should generally be attempted before referral to CAM.
Treatment with CAM requires prior
authorization.
Frequency: Per CAM therapy selected
Optimum duration: Should be based upon the physician’s clinical judgment and
demonstration by the patient of positive symptomatic and functional gains.
Practitioner provided CAM therapy is generally
not recommended on a maintenance basis.
6.4.4 DISTURBANCES
OF SLEEP are common in chronic pain. Although primary insomnia may
accompany pain as an independent co-morbid condition, it more commonly occurs
secondary to the pain condition itself. Exacerbations of pain often are
accompanied by exacerbations of insomnia; the reverse can also occur. Sleep
laboratory studies have shown disturbances of sleep architecture in pain
patients. Loss of deep slow-wave sleep and increase in light sleep occur and
sleep efficiency, the proportion of time in bed spent asleep, is decreased.
These changes are associated with patient reports of non-restorative sleep.
Many
chronic pain patients develop behavioral habits that exacerbate and maintain
sleep disturbances. Excessive time in bed, irregular sleep routine, napping,
low activity, and worrying in bed are all maladaptive responses that can arise
in the absence of any psychopathology. There is some evidence that behavioral
modification, such as patient education and group or individual counseling, can
be effective in reversing the effects of insomnia. Behavioral modifications are
easily implemented and can include:
6.4.4.1 Maintaining
a regular sleep schedule, retiring and rising at approximately the same time on
weekdays and weekends.
6.4.4.2 Avoiding
daytime napping.
6.4.4.3 Avoiding
caffeinated beverages after lunchtime
6.4.4.4 Making
the bedroom quiet and comfortable, eliminating disruptive lights, sounds,
television sets, and keeping a bedroom temperature of about 65°F.
6.4.4.5 Avoiding
alcohol or nicotine within two hours of bedtime.
6.4.4.6 Avoiding
large meals within two hours of bedtime.
6.4.4.7 Exercising
vigorously during the day, but not within two hours of bedtime, since this may
raise core temperature and activate the nervous system.
6.4.4.8 Associating
the bed with sleep and sexual activity only, using other parts of the home for
television, reading and talking on the telephone.
6.4.4.9 Leaving
the bedroom when unable to sleep for more than 20 minutes, retuning to the
bedroom when ready to sleep again.
These modifications should be undertaken before sleeping medication is
prescribed for
long term use.
6.4.5 INJECTIONS—THERAPEUTIC
When considering the use of injections in chronic pain management, the
treating physician must
carefully
consider the inherent risks and benefits.
Any continued use of injections should be monitored using objective
measures such as:
·
Return-to-work or
maintaining work status.
·
Fewer
restrictions at work or performing activities of daily living
·
Decrease in usage
of medications
Measurable
functional gains, such as increased range of motion for documented increase in
strength. Reduction of reported pain scores
6.4.5.1 Spinal
Therapeutic Injections General Description –The following injections are
considered to be reasonable treatment for patients with chronic pain. Other
injections not listed may be beneficial. Therapeutic spinal injections
typically may be used after initial conservative treatments, such as
physical and occupational therapy, medication, manual therapy, exercise,
acupuncture,
etc., have been undertaken.
Special Considerations – For all spinal injections (excluding trigger
point, botox and
occipital or peripheral nerve blocks)
multi-planar fluoroscopy, during procedures is required to document technique
and needle placement, and should be performed by a physician experienced in the
procedure. Permanent images are required to verify needle placement. The
subspecialty disciplines of the physicians may be varied, including, but not
limited to: anesthesiology, radiology, surgery, or physiatry. The practitioner
who performs injections for low back pain should document hands on training
through workshops of the type offered by organizations such as the
International Spine Intervention Society (ISIS) and/or completed fellowship
training with interventional training. Practitioners who perform spinal injections
must also be knowledgeable of radiation safety.
6.4.5.1.1 Epidural
Steroid Spinal Injections:
Description – Epidural steroid injections (ESI) deliver corticosteroid
into the epidural space. The purpose of ESI is to reduce pain and inflammation,
restoring range of
motion and thereby facilitating progress in
more active treatment programs. ESI uses
three approaches: transforaminal, translaminar (midline), and caudal. For ESI in
the low back, the transforaminal approach is the preferred method for
unilateral,
single-level pathology and for post-surgical patients. Also for the low back,
there is
good evidence that the transforaminal approach can deliver medication to the
target
tissue with few complications and can be used to identify the specific site of
pathology.
Needle Placement –Multi-planar fluoroscipic imaging is required for all
transforaminal
epidural steroid injections. Contrast epidurograms allow one to verify the flow
of
medication into the epidural space. Permanent images are required to verify
needle
placement. Indications – There is some evidence that epidural steroid
injections are
effective for patients with radicular pain or radiculopathy (sensory or motor
loss in a
specific dermatome or myotome). Although there is no evidence regarding the
effectiveness of ESI for non-radicular pain, it is a generally accepted
intervention.
Frequency: Up to 3 treatments (a treatment may be a one or two level
injection) over a
period of six months, depending upon each patient’s response.
Maximum: Two sessions (consisting of up to three injections each) may be done
in
one year based upon the patient’s response.
6.4.5.1.2 Zygapophyseal
(Facet) Injection: Description – A generally accepted intra-articular or
pericapsular injection of local anesthetic and corticosteroid. There is
conflicting evidence to support a long-term therapeutic effect using facet
injections. Indications patients with
pain suspected to
be of facet origin – Patients with recurrent pain should be evaluated,
to determine the
need for a rhizotomy.
Facet injections may be repeated if they result in documented functional
benefit and/
or at least an 50% initial improvement in pain as measured by accepted
pain scales (such as VAS). Maximum
Duration: 4 per level per year. Prior authorization must be obtained for
injections beyond three levels.
6.4.5.1.3 Sacro-iliac
Joint Injection: Description – A generally accepted injection of local
anesthetic in an intra-articular
fashion
into the sacro-iliac joint under radiographic guidance. May include the use of
corticosteroids. Long-term therapeutic effect has not yet been established.
Indications – Primarily diagnostic to rule out sacroiliac joint dysfunction vs.
other pain
generators. Intra-articular injection can be of value in diagnosing the
pain generator. These injections may be repeated if they result in increased
documented functional benefit and/or at least an 50% initial improvement in
pain scales as measured by accepted pain scales (such as VAS).
Maximum Duration: 3 injections per year.
6.4.5.2 Trigger
Point Injections: Description – Trigger point injection consists of dry
needling or injection of local anesthetic with or without corticosteroid into
highly localized, extremely sensitive bands of skeletal muscle fibers that
produce local and referred pain when activated. Medication is injected in the
area of maximum tenderness. Injection efficacy can be enhanced if injections
are immediately followed by myofascial therapeutic interventions, such as
vapo-coolant spray and stretch, ischemic pressure massage (myotherapy),
specific soft tissue mobilization and physical modalities.
The
effectiveness of trigger point injection is uncertain, in part due to the
difficulty of
demonstrating
advantages of active medication over injection of saline. Needling alone may be
responsible for some of the therapeutic response.
Indications – Trigger point injections may be used to relieve myofascial
pain and facilitate
active therapy and stretching of the affected areas. They are to be used as an
adjunctive
treatment in combination with other active treatment modalities. Trigger point
injections
should be utilized primarily for the purpose of facilitating functional
progress. Trigger point
injections are indicated in those patients where well-circumscribed trigger
points have
been consistently observed. Generally, these injections are not necessary
unless
consistently observed trigger points are not responding to specific,
noninvasive,
myofascial interventions within approximately a 4-week time frame.
Frequency: Weekly. Suggest no more than 4 injection sites per session
per week to avoid
significant post-injection soreness.
Optimum duration: 4 sessions.
Maximum duration: 8 weeks. Some patients may require 2 to 4 repetitions of
trigger point
injection series over a 1 to 2 year period.
6.4.5.3 Botulinum
Toxin (Botox) Injection: Description – Used to temporarily weaken or
paralyze muscles. May reduce muscle pain in conditions associated with
spasticity, dystonia, or other types of painful muscle spasm. Neutralizing
antibodies develop in at least 4% of patients treated with botulinum toxin type
A, rendering it ineffective. Several antigenic types of botulinum toxin have
been described. Botulinum toxin type B, first approved by the Food and Drug
Administration (FDA) in 2001, is similar pharmacologically to botulinum toxin
type A, and there is good evidence of its efficacy in improving function in
cervical dystonia (torticollis). It appears to be effective in patients who
have become resistant to the type A toxin. The immune responses to botulinum
toxins type A and B are not cross-reactive, allowing type B toxin to be used
when type A action is blocked by antibody. Experimental work with healthy human
volunteers suggests that muscle paralysis from type B toxin is not as complete
or as long lasting as that resulting from type A. The duration of treatment
effect of botulinum toxin
type B for cervical dystonia has been estimated to be 12 to 16 weeks.
EMG needle
guidance may permit more precise delivery of botulinum toxin to the
target area.
Indications – To improve range of motion and reduce painful muscle
spasm. May be useful
in musculoskeletal conditions associated
with muscle spasm or headaches. There should be evidence of limited range of
motion prior to the injection. May be useful in central neurologic conditions
that produce spasticity or dystonia (e.g., brain injury, spinal cord injury, or
stroke). Use is recommended according to current FDA guidelines.
Frequency: No less than 3 months between re-administration.
Optimum duration: 3 to 4 months.
Maximum duration: Currently unknown. Repeat injections should be based upon
functional improvement and therefore used sparingly in order to avoid
development of
antibodies that might render future injections ineffective.
6.4.6 MEDICATIONS
There
is no single formula for pharmacological treatment of patients with chronic
nonmalignant pain. Control of chronic non-malignant pain is expected to involve
the use of medication. Strategies for
pharmacological
control of pain cannot be precisely specified in advance. Rather, drug
treatment requires close monitoring of the patient’s response to therapy,
flexibility on the part of the prescriber and a willingness to change treatment
when circumstances change. Many of the drugs discussed in the medication
section were licensed for indications other than analgesia, but are effective
in the control of many types of chronic pain. Consensus regarding the use of
opioids has generally been reached in the field of cancer pain, where
nociceptive mechanisms are generally identifiable, expected survival may be
short, and symptomatic relief is emphasized more than functional outcomes. In
injured workers, by contrast, central and neuropathic mechanisms frequently
overshadow nociceptive processes, expected survival is relatively long, and
return to a high level of function is a major goal of treatment. Approaches to
pain, which were developed in
the
context of malignant pain, therefore may not be transferable to chronic
non-malignant pain. All medications should be given an appropriate trial in
order to test for therapeutic effect. Trials of medication requiring specific
therapeutic drug levels may take several months to achieve, depending upon the
half-life of the drug. It is recommended that patients with chronic
nonmalignant pain be maintained on drugs that have the least serious side
effects. For the clinician to interpret the following material, it should be
noted that: (1) drug profiles listed are not complete; (2) dosing of drugs will
depend upon the specific drug, especially for off-label use; and
(3) not all drugs within each class are
listed, and other drugs within the class may be appropriate. Clinicians should
refer to informational texts or consult a pharmacist before prescribing
unfamiliar medications or when there is a concern for drug interactions. The
following drug classes are listed in alphabetical order, not in order of
suggested use. The following list is not all inclusive. It is acknowledged that
medications not on this list may be appropriate choices for the care of injured
workers.
6.4.6.1 Alpha-Acting
Agents: Noradrenergic pain-modulating systems are present in the central
nervous system, and the alpha-2 adrenergic receptor may be involved in the
functioning of these pathways. Alpha-2 agonists may act by stimulating
receptors in the substantia gelatinosa of the dorsal horn of the spinal cord,
inhibiting the transmission of nociceptive signals. Spasticity may be reduced
by presynaptic inhibition of motor neurons. Given limited experience with their
use, they cannot be considered first-line analgesics, but a trial of their use
may be warranted in many cases of refractory pain.
6.4.6.1.1 Clonidine
(Catapres)
6.4.6.1.1.1 Description – Central alpha 2 agonist
6.4.6.1.1.2 Indications
– Sympathetically mediated pain, treatment of withdrawal from opioids.
6.4.6.1.1.3 Dosing and Time to Therapeutic Effect –
Increase dosage weekly to therapeutic effect.
6.4.6.1.1.4 Recommended Laboratory Monitoring – Renal
function.
6.4.6.1.2 Tizanidine
(Zanaflex)
6.4.6.1.2.1 Description
– Alpha 2 adrenergic agonist.
6.4.6.1.2.2 Indications
– Spasticity, musculoskeletal disorders.
6.4.6.1.2.3 Dosing and Time to Therapeutic Effect – As
needed (PRN) or titrate to effective dose.
6.4.6.1.2.4 Recommended
Laboratory Monitoring – Hepatic and renal function.
6.4.6.2 Anticonvulsants:
Although the mechanism of action of anticonvulsant drugs in neuropathic pain
states remains to be fully defined, they appear to act as nonselective sodium
channel blocking agents. A large variety of sodium channels are present in
nervous tissue, and some of these are important mediators of nociception, as
they are found primarily in unmyelinated fibers and their density increases
following nerve injury. While the pharmacodynamic effects of the various
anticonvulsant drugs are similar, the pharmacokinetic effects differ
significantly. Carbamazepine has important effects as an inducer of hepatic
enzymes and may influence the metabolism of other drugs enough to present
problems in patients taking more than one drug. Gabapentin and oxcarbazepine,
by contrast, are relatively non-significant enzyme inducers, creating fewer
drug interactions.
6.4.6.2.1 Gabapentin
(Neurontin)
6.4.6.2.1.1 Description
– Structurally related to gamma-aminobutyric acid (GABA) but does not interact
with GABA receptors.
6.4.6.2.1.2 Indications
– Neuropathic pain.
6.4.6.2.1.3 Dosing
and Time to Therapeutic Effect – Dosage may be increased over
several days.
6.4.6.2.1.4 Recommended Laboratory Monitoring – Renal function.
6.4.6.2.2 Oxcarbazepine (Trileptal)
|
6.4.6.2.2.1
|
Description – The mechanism
of action resembles that of carbamazepine, but
|
|
|
has an advantage in being a
less potent inducer of hepatic enzymes.
|
|
|
Controlled trials of its
effectiveness in chronic pain are lacking.
|
|
6.4.6.2.2.2
|
Indications – Neuropathic
pain.
|
|
6.4.6.2.2.3
|
Dosing and Time to
Therapeutic Effect – Dosage may be increased weekly.
|
|
6.4.6.2.2.4
|
Recommended Laboratory
Monitoring – Drug levels, renal and hepatic
|
function.
6.4.6.2.3 Carbamazepine (Tegretol)
6.4.6.2.3.1 Description – Anticonvulsant structurally
related to tricyclic antidepressants.
6.4.6.2.3.2 Indications – Trigeminal neuralgia and
other neuropathic pain.
6.4.6.2.3.3 Dosing and Time to Therapeutic Effect –
Dosage levels typically exceed those utilized for seizure prophylaxis. Titrate
to desired effect.
6.4.6.2.3.4 Recommended Laboratory Monitoring – Drug
levels, renal and hepatic function, complete blood count.
6.4.6.3 Antidepressants: are classified into a number of categories
based on their chemical structure and their effects on neurotransmitter
systems. Their effects on depression are attributed to their actions on
disposition of norepinephrine and serotonin at the level of the synapse;
although these synaptic actions are immediate, the symptomatic response in
depression is delayed by several weeks. When used for chronic pain, the effects
may in part arise from treatment of underlying depression, but may also involve
additional neuromodulatory effects on endogenous opioid systems, raising pain
thresholds at the level of the spinal cord.
Pain responses may occur at lower drug doses with shorter times to
symptomatic response than are observed when the same compounds are used in the
treatment of mood disorders. Neuropathic pain, diabetic neuropathy,
post-herpetic neuralgia, and cancer-related pain may respond to antidepressant
doses low enough to avoid adverse effects that often complicate the treatment
of depression.
6.4.6.3.1 Tricyclics
(e.g., amitriptyline [Elavil], nortriptyline [Pamelor, Aventyl], doxepin
[Sinequan, Adapin])
6.4.6.3.1.1 Description
– Serotonergics, typically tricyclic antidepressants (TCAs), are utilized for
their serotonergic properties as increasing CNS serotonergic tone can help
decrease pain perception in non-antidepressant dosages. Amitriptyline is known
for its ability to repair Stage 4 sleep architecture, a frequent problem found
in chronic pain patients and to treat depression, frequently associated with
chronic pain.
6.4.6.3.1.2 Indications
– Chronic musculoskeletal and/or neuropathic pain, insomnia. Second line drug
treatment for depression.
6.4.6.3.1.3 Dosing
and Time to Therapeutic Effect – Varies by specific tricyclic. Low dosages are
commonly used for chronic pain and/or insomnia.
6.4.6.3.1.4 Recommended Laboratory Monitoring – Renal
and hepatic function. EKG for those on high dosages or with cardiac risk.
6.4.6.3.2 Selective
serotonin reuptake inhibitors (SSRIs) (e.g., citalopram [Celexa], fluoxetine
[Prozac], paroxetine [Paxil], sertraline [Zoloft]).
6.4.6.3.2.1 Description
– SSRIs are characterized by the predominance of inhibition of serotonin
reuptake at the pre-synaptic nerve terminal.
6.4.6.3.2.2 Indications
– Depression, chronic pain with depression and/or anxiety.
6.4.6.3.2.3 Time
to Produce Therapeutic Effect – 3 to 4 weeks.
6.4.6.3.2.4 Recommended
Laboratory Monitoring – Renal and hepatic function.
6.4.6.3.3 Atypical
Antidepressants/Other Agents
6.4.6.3.3.1 Description
– Venlafaxine, (Effexor), nefazadone (Serzone), trazodone (Deseryl), and
mirtazapine (Remeron) share adjuvant analgesic effects with tricyclic
antidepressants. They differ in their side effect and drug interaction
profiles.
6.4.6.3.3.2 Indications
– Venlafaxine is approved for generalized anxiety disorder, bupropion for
smoking cessation.
6.4.6.3.3.3 Recommended Laboratory Monitoring – Drug
specific.
6.4.6.4 Hypnotics and
Sedatives: Sedative and hypnotic drugs decrease activity, induce
drowsiness, and moderate agitation. Many drugs produce these effects incidental
to their usual intended effects, similar to the side effects of many
antihistamines and antidepressants.
6.4.6.4.1 Zaleplon
(Sonata)
6.4.6.4.1.1 Description
– A nonbenzodiazepine hypnotic.
6.4.6.4.1.2 Indications
– Insomnia.
6.4.6.4.1.3 Dosing
and Time to Therapeutic Effect – Time of onset is 30 to 60 minutes. Due to
rapid elimination, may be taken as little as 4 hours before awakening.
6.4.6.4.1.4 Recommended
Laboratory Monitoring – Hepatic function.
6.4.6.4.2 Zolpidem
(Ambien)
6.4.6.4.2.1 Description
– A nonbenzodiazepine hypnotic, which does not appear to cause rebound
insomnia. It has little respiratory depression and insignificant anxiolytic or
muscle relaxant activity.
6.4.6.4.2.2 Indications
– Short-term use for insomnia
6.4.6.4.2.3 Time
to Therapeutic Effect – Onset of action is 30 to 60 minutes
6.4.6.4.2.4 Recommended
Laboratory Monitoring – Hepatic function.
6.4.6.5 Skeletal
Muscle Relaxants: are most useful for acute musculoskeletal injury or
exacerbation of injury.
6.4.6.5.1 Cyclobenzaprine
(Flexeril)
6.4.6.5.1.1 Description
– Structurally related to tricyclics.
6.4.6.5.1.2 Indications
– Chronic pain associated with muscle spasm.
6.4.6.5.1.3 Dosing
and Time to Therapeutic Effect – Variable, onset of action is 1 hour.
6.4.6.5.1.4 Recommended Laboratory Monitoring –
Hepatic and renal function.
6.4.6.5.2 Carisoprodol
(Soma)
6.4.6.5.2.1 Description
– Mode of action may be central; meprobamate is an active metabolite.
6.4.6.5.2.2 Indications
– Chronic pain associated with muscle spasm.
6.4.6.5.2.3 Recommended Laboratory Monitoring – Renal
and hepatic function.
6.4.6.5.3 Metazalone
(Skelaxin)
6.4.6.5.3.1 Description
– Central acting muscle relaxant.
6.4.6.5.3.2 Indications
– Muscle spasm.
6.4.6.5.3.3 Dosing
and Time to Therapeutic Effect – Onset of action 1 hour.
6.4.6.5.3.4 Recommended
Laboratory Monitoring – Hepatic function.
6.4.6.6 Opioids:
are the most powerful analgesics. Opioids include some of the oldest and most
effective drugs used in the control of severe pain. The discovery of opioids
receptors and their endogenous peptide ligands has led to an understanding of
effects at the binding sites of these naturally occurring substances. Most of
their analgesic effects have been attributed to their modification of activity
in pain pathways within the central nervous system; however, it has become
evident that they also are active in the peripheral nervous system. Activation
of receptors on the peripheral terminals of primary afferent nerves can mediate
antinociceptive effects, including inhibition of neuronal excitability and
release of inflammatory peptides. Some of their undesirable effects on
inhibiting gastrointestinal motility are peripherally mediated by receptors in
the bowel wall. The central nervous
system actions of these drugs account for much of their analgesic effect.
Consultation or referral to a pain specialist should be considered when the
pain persists but the underlying tissue pathology is minimal or absent and
correlation between the original injury and the severity of impairment is not
clear. Consider consultation if suffering and pain behaviors are present and
the patient continues to request medication, or when standard treatment
measures have not been successful or are not indicated.
6.4.6.6.1 On-Going,
Long-Term Management – Actions may include:
6.4.6.6.1.1 Prescriptions
from a single practitioner,
6.4.6.6.1.2 Ongoing
review and documentation of pain relief, functional status, appropriate
medication use, and side effects,
6.4.6.6.1.3 Ongoing
effort to gain improvement of social and physical function as a result of pain
relief,
6.4.6.6.1.4 Contract
detailing reasons for termination of supply, with appropriate tapering of dose,
6.4.6.6.1.5 Use
of random drug screening as deemed appropriate by the prescribing physician,
6.4.6.6.1.6 Use
of more than two opioids: a long acting opioid for maintenance of pain relief
and a short acting opioid for limited rescue use when pain exceeds the routine
level. If more than two opioids are prescribed for long-term use, a second
opinion from specialist who is Board Certified in Neurology, Physical Medicine
and Rehabilitation, or Anesthesiology with recognized training and/or
certification in pharmacological pain management is strongly recommended.
6.4.6.6.1.7 Use
of acetaminophen-containing medications in patients with liver disease should
be limited; and
6.4.6.6.1.8 Continuing
review of overall situation with regard to nonopioid means of pain control.
6.4.6.7 Nonsteroidal
Anti-Inflammatory Drugs: Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) are
useful for pain and inflammation. In mild cases, they may be the only drugs
required for analgesia. There are several classes of NSAIDs and the response of
the individual injured worker to a specific medication is
unpredictable.
For this reason a range of NSAIDs may be tried in each case with the most
effective preparation being continued.
6.4.6.7.1 Non-selective
Nonsteroidal Anti-Inflammatory Drugs
6.4.6.7.2 Selective
Cyclo-oxygenase-2 (COX-2) Inhibitors COX-2 inhibitors are more recent NSAIDs
and differ in adverse side effect profiles from the traditional NSAIDs. The
major advantages of selective COX-2 inhibitors over traditional NSAIDs are that
they have less gastrointestinal toxicity and no platelet
effects. COX-2 inhibitors can worsen renal function in patients with
renal insufficiency; thus, renal function may need monitoring.
6.4.6.8 Topical Drug
Delivery:
6.4.6.8.1 Description
– Topical medications may be an alternative treatment for localized
musculoskeletal disorders and is an acceptable form of treatment in selected.
6.4.6.8.2 Indications
– Generalized musculoskeletal or joint pain. Patient selection must be rigorous
to select those patients with the highest probability of compliance.
6.4.6.8.3 Dosing and Time to Therapeutic Effect – It is
necessary that all topical agents be used with strict instructions for
application as well as maximum number of applications per day to obtain the
desired benefit and avoid potential toxicity.
6.4.6.9 Other Agents:
6.4.6.9.1 Tramadol
(Ultram)
6.4.6.9.1.1 Description
– An opioid partial agonist that is generally well tolerated, does not cause GI
ulceration, or exacerbate hypertension or congestive heart failure.
6.4.6.9.1.2 Indications
– Mild to moderate pain relief. This drug has been shown to provide pain relief
equivalent to that of commonly prescribed NSAIDs.
6.4.6.9.2 Baclofen
(Lioresal)
6.4.6.9.2.1 Description
– May be effective due to stimulation of Gamma Aminobutyric Acid (GABA)
receptors.
6.4.6.9.2.2 Indications
– Pain from muscle rigidity.
6.4.6.9.2.3 Recommended Laboratory Monitoring –
Renal function.
6.4.6.9.3 Mexilitene
(Mexitil)
6.4.6.9.3.1 Description
– An antiarrhythmic drug, which, like some anticonvulsive agents, may act on
ion channels in neuronal tissue and reduce its pathological activity to a more
stable level. Low concentrations may suffice to abolish impulses in damaged
nerves, and mexilitene has been used successfully to treat neuropathic pain.
6.4.6.9.3.2 Indications
– Neuropathic pain.
6.4.6.9.3.3 Recommended
Laboratory Monitoring – Hepatic function, CBC. Plasma levels may also be
necessary.
6.4.7 ORTHOTICS/PROSTHETICS/EQUIPMENT
Devices and adaptive equipment may be necessary in order to reduce
impairment and disability, to facilitate medical recovery, to avoid
re-aggravation of the injury, and to maintain maximum medical improvement.
Indications would be to provide relief of the industrial injury or prevent
further injury and include the need to control neurological and orthopedic
injuries for reduced stress during functional activities. In addition, they may
be used to modify tasks through instruction in the use of a device or physical
modification of a device. Equipment needs may need to be reassessed
periodically.
Equipment may include high and low
technology assistive devices, computer interface or seating, crutch or walker
training, and self-care aids. It should improve safety and reduce risk of
re-injury. Standard equipment to alleviate the effects of the injury on the
performance of activities of daily living may vary from simple to complex
adaptive devices to enhance independence and safety. Certain equipment related
to cognitive impairments may also be required.
Ergonomic modifications may be necessary to
facilitate medical recovery, to avoid re-aggravation of the injury, and to
maintain maximum medical improvement. Ergonomic evaluations with subsequent
recommendations may assist with the patients’ return-to-work.
For chronic pain disorders, equipment such
as foot orthoses or lumbar support devices may be helpful. The injured worker
should be educated as to the potential harm from using a lumbar support for a
period of time greater than which is prescribed. Special cervical orthosis and/or
equipment may have a role in the rehabilitation of a cervical injury such as
those injuries to a cervical nerve root resulting in upper extremity weakness
or a spinal cord injury with some degree of paraparesis or tetraparesis.
Fabrication/modification of orthotics, including splints, would be used when
there is need to normalize weight-bearing, facilitate better motion response,
stabilize a joint with insufficient muscle or proprioceptive/reflex
competencies, to protect subacute conditions
as
needed during movement, and correct biomechanical problems.
Orthotic/prosthetic training is the skilled instruction (preferably by
qualified providers) in the proper
use of orthotic devices and/or prosthetic limbs.
For information regarding specific types of
orthotics/prosthetics/equipment, refer to individual medical treatment
guidelines.
6.4.8 PERSONALITY/PSYCHOLOGICAL/PSYCHOSOCIAL
INTERVENTION Psychosocial treatment is a generally accepted,
well-established therapeutic and diagnostic procedure with selected use in
acute pain problems, but with more widespread use in sub-acute and chronic pain
populations. Psychosocial treatment may be important component in the total
management of a patient with chronic pain and should be implemented as soon as
the problem is identified.
Once a diagnosis consistent with the
standards of the American Psychiatric Association Diagnostic Statistical Manual
of Mental Disorders has been determined, the patient should be evaluated for
the potential need for psychiatric medications. Use of any medication to treat
a diagnosed condition may be ordered by the authorized treating physician or by
the consulting psychiatrist. Visits for management of psychiatric medications
are medical in nature and are not a component of psychosocial treatment.
Therefore, separate visits for medication management may be necessary,
depending upon the patient and medications selected.
The screening or diagnostic workup should
have clarified and distinguished between pre-existing, aggravated, and/or
purely causative psychological conditions. Therapeutic and diagnostic
modalities include, but are not limited to, individual counseling, and group
therapy. Treatment can occur within an individualized model, a
multi-disciplinary model, or within a structured pain management program.
A psychologist with a Ph.D., PsyD, EdD
credentials, or a Psychiatric MD/DO may perform psychosocial treatments. Other
licensed mental health providers working in consultation with a Ph.D., PsyD,
EdD, or Psychiatric MD/DO, and with experience in treating chronic pain
disorders in injured workers may also perform treatment.
Frequency: 1 to 5 times weekly for the first
4 weeks (excluding hospitalization, if required), decreasing to 1 to 2 times
per week for the second month. Thereafter, 2 to 4 times monthly with the
exception of exacerbations which may require increased frequency of visits. Not
to include visits for medication management.
Maximum duration: 6 to 12 months, not to
include visits for medication management. For select patients, longer
supervised treatment may be required.
6.4.9 RESTRICTION
OF ACTIVITIES Continuation of normal daily activities is the goal for
chronic pain patients since immobility will negatively affect rehabilitation.
Prolonged immobility results in a wide range of deleterious effects, such as a
reduction in aerobic capacity and conditioning, loss of muscle strength and
flexibility, increased segmental stiffness, promotion of bone demineralization,
impaired disc nutrition, and the facilitation of the illness role.
6.4.10 RETURN-TO-WORK is one
of the major components in chronic pain management. REHABILITATION – It is
understood Individuals with Chronic Pain may require additional visits due to
acute exacerbations. The practitioner is required to document the rationale for
care and may be subject to Utilization Review. All visit limits pertain to an
annual amount. It
is also understood that practitioners should
only provide treatment that is consistent with impairments and dysfunctions
identified by a comprehensive physical assessment.
6.4.11 THERAPY–ACTIVE
therapies are based on the philosophy that therapeutic exercise and/or
activity are beneficial for restoring flexibility, strength, endurance,
function, range of motion, and alleviating discomfort. Active therapy requires
an internal effort by the individual to complete a specific exercise or task,
and thus assists in developing skills promoting independence to allow self-care
after discharge. This form of therapy requires supervision from a therapist or
medical provider such as verbal, visual, and/or tactile instructions. At times
a provider may help stabilize the patient or guide the movement pattern but the
energy required to complete the task is predominately executed by the patient.
Patients should be instructed to continue
active therapies at home as an extension of the treatment process in order to
maintain improvement levels. Home exercise can include exercise with or without
mechanical assistance or resistance and functional activities with assistive
devices. Interventions are selected based on the complexity of the presenting
dysfunction with ongoing examination, evaluation and modification of the plan
of care as improvement or lack thereof occurs. Change and/or discontinuation of
an intervention should occur if there is attainment of expected goals/outcome,
lack of progress, lack of tolerance and/or lack of motivation. Passive
interventions/modalities may only be used as adjuncts to the active program.
6.4.11.1 Activities
of Daily Living: Supervised instruction, active-assisted training, and/or
adaptation of activities or equipment to improve a person’s capacity in normal
daily living activities such as self-care, work re-integration training,
homemaking, and driving.
6.4.11.2 Functional
Activities: are the use of therapeutic activity to enhance mobility, body
mechanics, employability, coordination, and sensory motor integration.
6.4.11.3 Nerve
Gliding: exercises consist of a series of flexion and extension movements
of the hand, wrist, elbow, shoulder, and neck that produce tension and
longitudinal movement along the length of the median and other nerves of the
upper extremity. These exercises are based on the principle that the tissues of
the peripheral nervous system are designed for movement, and that tension and
glide (excursion) of nerves may have an effect on neurophysiology through
alterations in vascular and axoplasmic flow. Biomechanical principles have been
more thoroughly studied than clinical outcomes.
6.4.11.4 Neuromuscular
Re-education: is the skilled application of exercise with manual,
mechanical, or electrical facilitation to enhance strength, movement patterns,
neuromuscular response, proprioception, kinesthetic sense, coordination
education of movement, balance, and posture. Indications include the need to
promote neuromuscular responses through carefully timed proprioceptive stimuli,
to elicit and improve motor activity in patterns similar to normal
neurologically developed sequences, and improve neuromotor response with
independent control.
Maximum number of visits 24
6.4.11.5 Proper
Work Techniques: Please refer to the “Job Site Evaluation” and “Job Site
Alteration” sections of these guidelines.
6.4.11.6 Therapeutic
Exercise: with or without mechanical assistance or resistance may include
isoinertial, isotonic, isometric and isokinetic types of exercises. Indications
include the need for cardiovascular fitness, reduced edema, improved muscle
strength, improved connective tissue strength and integrity, increased bone
density, promotion of circulation to enhance soft tissue healing, improvement
of muscle recruitment, increased range of motion, and are used to promote
normal movement patterns. Can also include complementary/alternative exercise
movement therapy.
Time
to produce effect: 2 to 6 treatments
Frequency: 3 to 5 times per week
Optimum duration: 4 to 8 weeks
Maximum duration: 24 visits, and maximum of 24 in combination with functional activities
6.4.12 THERAPY
— PASSIVE Most of the following passive therapies and modalities are
generally accepted methods of care for a variety of work-related injuries.
Passive therapy includes those treatment modalities that do not require energy
expenditure on the part of the patient. They are principally effective during
the early phases of treatment and are directed at controlling symptoms such as
pain, inflammation and swelling and to improve the rate of healing soft tissue injuries.
They should be used adjunctively with active therapies such as postural
stabilization and exercise programs to help control swelling, pain, and
inflammation during the active rehabilitation process. Please refer to Section
B. 4. General Guideline Principles, Active Interventions. Passive therapies may
be used intermittently as a provider deems appropriate or regularly if there
are specific goals with objectively measured functional improvements during
treatment.
On occasion, specific diagnoses and
post-surgical conditions may warrant durations of treatment beyond those listed
as "maximum.” factors such as exacerbation of symptoms, re-injury,
interrupted continuity of care, and comorbidities may also extend durations of
care. Specific goals with objectively measured functional improvement during
treatment must be cited to justify extended durations of care. It is
recommended that, if no functional gain is observed after the number of
treatments under “time to produce effect” have been completed, alternative
treatment interventions, further diagnostic studies, or further consultations
should be pursued.
The following passive therapies are listed
below:
6.4.12.1 Electrical
Stimulation (Unattended and Attended): is an accepted treatment. Once
applied, unattended electrical stimulation requires minimal on-site supervision
by the provider. Indications include pain, inflammation, muscle spasm, atrophy,
decreased circulation, and the need for osteogenic stimulation. A home unit
should be purchased if treatment is effective and frequent use is recommended.
Time to produce effect: 2 to 4
treatments Maximum duration: 14 visits
6.4.12.2 Iontophoresis:
is an accepted treatment which consists of the transfer of medication,
including, but not limited to, steroidal anti-inflammatories and anesthetics,
through the use of electrical stimulation. Indications include pain
(Lidocaine), inflammation (hydrocortisone, salicylate), edema (mecholyl,
hyaluronidase, salicylate), ischemia (magnesium, mecholyl, iodine), muscle
spasm (magnesium, calcium), calcific deposits (acetate), scars, and keloids
(sodium chloride, iodine, acetate). There is no proven benefit for this therapy
in the low back
Time
to produce effect: 1 to 4 treatments Frequency: 3 times per week with at least
48 hours between treatments Maximum duration: 8 visits per body region
6.4.12.3 Manipulation:
is generally accepted, well-established and widely used therapeutic
intervention for low back pain. Manipulative Treatment (not therapy) is defined
as the therapeutic application of manually guided forces by an operator to
improve physiologic function and/or support homeostasis that has been altered
by the injury or occupational disease, and has associated clinical
significance.
High velocity, low amplitude (HVLA)
technique, chiropractic manipulation, osteopathic manipulation, muscle energy
techniques, counter strain, and non-force techniques are all types of
manipulative treatment. This may be applied by osteopathic physicians (D.O.),
chiropractors (D.C.), properly trained physical therapists (P.T.), or properly
trained medical physicians. Under these different types of manipulation exist
many subsets of different techniques that can be described as a) direct- a
forceful engagement of a restrictive/ pathologic barrier, b) indirect- a
gentle/non-forceful disengagement of a restrictive/ pathologic barrier, c) the
patient actively assists in the treatment and d) the patient relaxing, allowing
the practitioner to move the body tissues. When the proper diagnosis is made
and coupled with the appropriate technique, manipulation has no
contraindications and can be applied to all tissues of the body. Pre-treatment
assessment should be performed as part of each manipulative treatment visit to
ensure that the correct diagnosis and correct treatment is employed.
High velocity, low amplitude (HVLA)
manipulation is performed by taking a joint to its end range of motion and
moving the articulation into the zone of accessory joint movement, well within
the limits of anatomical integrity. There is good scientific evidence to
suggest that HVLA manipulation can be helpful for patients with acute low back
pain problems without radiculopathy when used within the first 4 to 6 weeks of
symptoms. Although the evidence for sub-acute and chronic low back pain and low
back pain with radiculopathy is less convincing, it is a generally accepted and
well-established intervention for these conditions. Indications for
manipulation include joint pain, decreased joint motion, and joint adhesions.
Contraindications to HVLA manipulation include joint instability, fractures,
severe
osteoporosis, infection, metastatic cancer, active inflammatory arthritides,
aortic
aneurysm, and signs of progressive neurologic deficits.
Time
to produce effect for all types of manipulative treatment: 1 to 6 treatments.
Frequency: Up to 3 times per week for the first 4 weeks as indicated by the
severity of
involvement and the desired effect, then up to 2 treatments per week for the
next 4 weeks.
For further treatments, twice per week or less to maintain function.
Maximum
duration: 26 visits.
The combination of 97140 plus either CMT or OMT code is equal to one visit when
performed on the same day. Any combination of manual therapeutic intervention
exceeding 26 visits (not units) need to go to UR.
6.4.12.4 Massage
— Manual or Mechanical: Massage is manipulation of soft tissue with broad
ranging relaxation and circulatory benefits. This may include techniques that
include pressing, lifting, rubbing, pinching of soft tissues by, or with, the
practitioner's hands. Indications include edema (peripheral or hard and
non-pliable edema), muscle spasm, adhesions, the need to improve peripheral
circulation and range of motion, or to increase muscle relaxation and
flexibility prior to exercise.
In
sub-acute low back pain populations there is good evidence that massage can
increase function when combined with exercise and patient education. Some
studies have demonstrated a decrease in provider visits and pain medication use
with combined therapy. One study indicated improved results with acupressure
massage. It is recommended that all massage be performed by trained,
experienced therapists and be accompanied by an active exercise program and
patient education. In contrast to the subacute population, massage is a
generally accepted treatment for the acute low back pain population, although no
studies have demonstrated its efficacy for this set of patients.
Time
to produce effect: Immediate Frequency: 1 to 3 times per week Maximum duration:
12 visits (CPT codes 97124 and 97140 can not exceed 26 visits in
combination).
6.4.12.5 Mobilization
(Joint): is a generally well-accepted treatment. Mobilization is passive
movement involving oscillatory motions to the vertebral segment(s). The passive
mobility is performed in a graded manner (I, II, III, IV, or V), which depicts
the speed and depth of joint motion during the maneuver. For further discussion
on Level V joint mobilization please see section on HVLA manipulation [Refer to
section 12. d.]. It may include skilled manual joint tissue stretching.
Indications include the need to improve joint play, segmental alignment,
improve intracapsular arthrokinematics, or reduce pain associated with tissue
impingement. Mobilization should be accompanied by active therapy. For Level V
mobilization contraindications include joint instability, fractures, severe
osteoporosis, infection, metastatic cancer, active inflammatory arthritides,
aortic aneurysm, and signs of progressive neurologic deficits. Time to produce
effect for all types of manipulative treatment: 1 to 6 treatments. Frequency:
Up to 3 times per week for the first 4 weeks as indicated by the severity of
involvement and the desired effect, then up to 2 treatments per week for the
next 4 weeks. For further treatments, twice per week or less to maintain
function. Maximum duration: 26 visits.
CPT codes 97124 and 97140 can not exceed 48 visits in
combination
6.4.12.6 Mobilization
(Soft Tissue): is a generally well-accepted treatment. Mobilization of soft
tissue is the skilled application of muscle energy, strain/counter strain,
myofascial release, manual trigger point release, and manual therapy techniques
designed to improve or normalize movement patterns through the reduction of
soft tissue pain and restrictions. These can be interactive with the patient
participating or can be with the patient relaxing and letting the practitioner
move the body tissues. Indications include muscle spasm
around
a joint, trigger points, adhesions, and neural compression. Mobilization should
be
accompanied
by active therapy.
Maximum duration: 26 visits CPT codes 97124 and 97140 can not exceed 48 visits
in
combination.
6.4.12.7 Short-Wave
Diathermy: is an accepted treatment which involves the use of equipment
that exposes soft tissue to a magnetic or electrical field. Indications include
enhanced collagen extensibility before stretching, reduced muscle guarding,
reduced inflammatory response, and enhanced re-absorption of
hemorrhage/hematoma or edema. It is an accepted modality as an adjunct to
acupuncture or situation where other forms of contact superficial heat is
contraindicated.
6.4.12.8 Superficial
Heat and Cold Therapy (excluding Infrared Therapy): is a generally accepted
treatment. Superficial heat and cold are thermal agents applied in various
manners that lower or raise the body tissue temperature for the reduction of
pain, inflammation, and/or effusion resulting from injury or induced by
exercise. Includes application of heat just above the surface of the skin at
acupuncture points. Indications include acute pain, edema and hemorrhage, need
to increase pain threshold, reduce muscle spasm, and promote
stretching/flexibility. Cold and heat packs can be used at home as an extension
of therapy in the clinic setting.
Time
to produce effect: Immediate
Frequency: 2 to 5 times per week
Maximum duration: 12 visits, with a maximum of 1 unit per day
6.4.12.9 Traction—Mechanical:
Traction modalities are contraindicated in patients with tumor, infections,
fracture, or fracture dislocation. Non-oscillating inversion traction methods
are contraindicated in patients with glaucoma or hypertension. Motorized
traction devices are included (i.e. VAX-D, DRX9000, etc.)
Time
to produce effect: 1 to 3 sessions up to 30 minutes. If response is negative
after 3
treatments,
discontinue this modality. Frequency: 2 to 3 times per week. A home traction
unit can be purchased if therapy proves effective. Maximum duration: 16 visits
6.4.12.10 Transcutaneous
Electrical Nerve Stimulation (TENS): is a generally accepted treatment.
TENS should include at least one instructional session for proper application
and use. Indications include muscle spasm, atrophy, and decreased circulation
and pain control. Minimal TENS unit parameters should include pulse rate, pulse
width and amplitude modulation. Consistent, measurable functional improvement
should be documented prior to the purchase of a home unit.
Time to produce effect: Immediate Frequency: Variable Duration: 3 visits
6.4.12.11 Ultrasound
(Including Phonophoresis): is an accepted treatment. Ultrasound uses sonic
generators to deliver acoustic energy for therapeutic thermal and/or
non-thermal soft tissue effects. Indications include scar tissue, adhesions,
collagen fiber and muscle spasm, and the need to extend muscle tissue or
accelerate the soft tissue healing. Ultrasound with electrical stimulation is
concurrent delivery of electrical energy that involves dispersive electrode
placement. Indications include muscle spasm, scar tissue, pain modulation, and
muscle facilitation.
Phonophoresis
is the transfer of medication to the target tissue to control inflammation and
pain through the use of sonic generators. These topical medications include,
but are not limited to, steroidal anti-inflammatory and anesthetics. Phonopheresis
is not recommended for Low Back Pain.
Time
to produce effect: 6 to 15 treatments
Frequency: 3 times per week
Maximum duration: 18 visits
6.4.13 THERAPY—ACTIVE The
following active therapies are widely used and accepted methods of care for a
variety of work-related injuries. They are based on the philosophy that
therapeutic exercise and/or activity are beneficial for restoring flexibility,
strength, endurance, function, range of motion, and can alleviate discomfort.
Active therapy requires an internal effort by the individual to complete a
specific exercise or task. This form of therapy requires supervision from a
provider such as verbal, visual, and/or tactile instruction(s). At times, the
provider may help stabilize the patient or guide the movement pattern but the
energy required to complete the task is predominately executed by the patient.
Patients
should be instructed to continue active therapies at home as an extension of
the treatment process in order to maintain improvement levels. Follow-up visits
to reinforce and monitor progress and proper technique are recommended. Home
exercise can include exercise with or without mechanical assistance or
resistance and functional activities with assistive devices. The following
active therapies are listed in alphabetical order:
6.4.13.1 Activities
of Daily Living (ADL) are well-established interventions which involve
instruction, active-assisted training, and/or adaptation of activities or
equipment to improve a person's capacity in normal daily activities such as
self-care, work re-integration training, homemaking, and driving.
Time to produce effect: 4 to 5 treatments Maximum duration: 10 visits
6.4.13.2 Aquatic
Therapy: is a well-accepted treatment which consists of the therapeutic use
of aquatic immersion for therapeutic exercise to promote strengthening, core
stabilization, endurance, range of motion, flexibility, body mechanics, and
pain management. Aquatic therapy includes the implementation of active therapeutic
procedures in a swimming or therapeutic pool. The water provides a buoyancy
force that lessens the amount of force gravity applies to the body. The
decreased gravity effect allows the patient to have a mechanical advantage and
more likely have a successful trial of therapeutic exercise. The therapy may be
indicated for individuals who:
Cannot tolerate active land-based or full-weight bearing therapeutic
procedures require increased support in the presence of proprioceptive deficit; Are at risk of compression fracture due to decreased bone density; have
symptoms that are exacerbated in a dry environment;
Would have a higher probability of meeting active therapeutic goals than
in a land-based environment. The pool should be large enough to allow full extremity range of motion and
fully erect posture. Aquatic vests, belts and other devices can be used to provide
stability, balance, buoyancy, and resistance.
Time to produce effect: 4 to 5 treatments Frequency: 3 to 5 times per
week Maximum
duration: 16 visits A self-directed program is recommended after the
supervised aquatics program has been established, or, alternatively a
transition to a land-based environment exercise program.
6.4.13.3 Functional
Activities: are well-established interventions which involve the use of
therapeutic activity to enhance mobility, body mechanics, employability,
coordination, balance, and sensory motor integration.
Time
to produce effect: 4 to 5 treatments Frequency: 3 to 5 times per week Maximum
duration: 26 visits Total number of visit 97110 and 97530 should not exceed 40
visits without pre
auhorization.
6.4.13.4 Functional
Electrical Stimulation: is an accepted treatment in which the application of
electrical current to elicit involuntary or assisted contractions of atrophied
and/or impaired muscles. It may be indicated for impaired muscle function to
radiculopathy. (Foot drop)
Time to produce effect: 2 to 6 treatments Frequency: 3 times per week
Maximum duration: 14 visits inclusive of electrical stimulation codes. If
beneficial, provide with home unit.
6.4.13.5 Neuromuscular
Re-education: is a generally accepted treatment. It is the skilled application
of exercise with manual, mechanical, or electrical facilitation to enhance
strength; movement patterns; neuromuscular response; proprioception,
kinesthetic sense, coordination; education of movement, balance, and posture.
Indications include the need to promote neuromuscular responses through
carefully timed proprioceptive stimuli, to elicit and improve motor activity in
patterns similar to normal neurologically developed sequences, and improve
neuromotor response with independent control.
Time to produce effect: 2 to 6 treatments
Frequency: 3-5 times per week
Maximum duration: 24 visits
6.4.13.6 Therapeutic
Exercise: is a generally well-accepted treatment. Therapeutic exercise, with or
without mechanical assistance or resistance, may include isoinertial, isotonic,
isometric and isokinetic types of exercises. Indications include the need for
cardiovascular fitness, reduced edema, improved muscle strength, improved
connective tissue strength and integrity, increased bone density, promotion of
circulation to enhance soft tissue healing, improvement of muscle recruitment,
improved proprioception, and coordination, increased range of motion.
Therapeutic exercises are used to promote normal movement patterns, and can
also include complementary/alternative exercise movement therapy (with
oversight of a physician or appropriate healthcare professional).
6.4.13.7 Spinal
Stabilization: is a generally well-accepted treatment. The goal of this
therapeutic program is to strengthen the spine in its neural and anatomic
position. The stabilization is dynamic which allows whole body movements while
maintaining a stabilized spine. It is the ability to move and function normally
through postures and activities without creating undue vertebral stress
Time to produce effect: 2 to 6 treatments Frequency: 3
to 5 times per week Maximum duration: 26 visits Total number of visits of 97110
& 97530 may not exceed 40 visits without pre-
authorization.
When considering operative intervention in
chronic pain management, the treating physician must carefully consider the
inherent risk and benefit of the procedure. All operative intervention should
be based on a positive correlation with clinical findings, the clinical course,
and diagnostic tests. A comprehensive assessment of these factors should have
led to a specific diagnosis with positive identification of the pathologic
condition. Surgical procedures are seldom meant to be curative and would be
employed in conjunction with other treatment modalities for maximum functional
benefit. Functional benefit should be objectively measured and includes the
following:
·
Return-to-work or
maintaining work status.
·
Fewer
restrictions at work or performing activities of daily living.
·
Decrease in usage
of medications.
·
Measurable
functional gains, such as increased range of motion or documented increase in
strength.
·
Education of the
patient should include the proposed goals of the surgery, expected gains, risks
or complications, and alternative treatment.
7.1 NEUROSTIMULATION
7.1.1 Description
— Neurostimulation is the delivery of low-voltage electrical stimulation to the
spinal cord or peripheral nerves to inhibit or block the sensation of pain.
This is a generally accepted procedure that has limited use. May be most
effective in patients with chronic, intractable limb pain who have not achieved
relief with oral medications, rehabilitation therapy, or therapeutic nerve
blocks,
and in whom the pain has persisted for longer than 6 months. Particular
technical expertise is required to perform this procedure and is available in
some neurosurgical, rehabilitation, and anesthesiology training programs and
fellowships. Physicians performing this procedure must be experienced in neurostimulation
implantation and participate in ongoing injection training workshops, such as
those sponsored by the Internal Society for Injection Studies or as sponsored
by implant manufacturers.
7.1.2 Indications
— Failure of conservative therapy including active and/or passive therapy,
medication management, or therapeutic injections. Habituation to narcotic
analgesics in the absence of a history of addictive behavior does not preclude
the use of neurostimulation. Only patients who meet the following criteria
should be considered candidates for neurostimulation:
7.1.2.1 A
diagnosis of a specific physical condition known to be chronically painful has
been made on the basis of objective findings; and
7.1.2.2 All
reasonable non-surgical treatment has been exhausted; and
7.1.2.3 Pre-surgical
psychiatric or psychological evaluation has been performed and has demonstrated
motivation and long-term commitment without issues of secondary gain; and
7.1.2.4 There
is no evidence of addictive behavior. (Tolerance and dependence to narcotic
analgesics are not addictive behaviors and do not preclude implantation.); and
7.1.2.5 The
topography of pain and its underlying pathophysiology are amenable to
stimulation coverage; and
7.1.2.6 A
successful neurostimulation screening test of 2-3 days. A screening test is
considered successful if the patient (a) experiences a 50% decrease in pain,
which may be confirmed by visual analogue scale (VAS.
7.1.2.7 For
spinal cord stimulation, a temporary lead is implanted and attached to an
external source to validate therapy effectiveness.
7.1.3 Operative
Treatment – Implantation of stimulating leads connected by extensions to either
an implanted neurostimulator or an implanted receiver powered by an external
transmitter. The procedure may be performed either as an open or a percutaneous
procedure, depending on the presence of epidural fibrosis and the anatomical
placement required for optimal efficacy.
7.1.4 Post-Operative
Considerations – MRI is contraindicated after placement of neurostimulators.
7.1.5 A
mandatory second opinion is required to confirm the rationale for the procedure
for non malignant pain.
7.2 INTRATHECAL
DRUG DELIVERY
7.2.1 Description
-This mode of therapy delivers small doses of medications directly into the cerebrospinal
fluid. Clinical studies are conflicting regarding long-term, effective pain
relief in patients with non-malignant pain. As with other routes of drug
administration, escalation of dose may be required. Typically, pump refills are
needed every 2-3 months.
7.2.2 General
Indications – It may be considered only in rare cases where all other commonly
used methods to control pain have failed and must be based on the
recommendation of at least one physician experienced in chronic pain management
in consultation with the primary treating physician. Patients should only be
selected for intrathecal drug delivery if they have opioid-responsive pain but
cannot tolerate the effects of systemic administration. The patient must have
good to excellent pain relief with a test dose prior to pump implantation. The
patient must be motivated for the procedure, and must understand the potential
for complications and requirements of treatment maintenance.
7.2.3 Surgical
Indications – Failure of conservative therapy including active and/or passive
therapy, medication management, or therapeutic injections. Only patients who
meet the following criteria should be considered candidates for intraspinal
analgesic infusions:
7.2.3.1 A
diagnosis of a specific physical condition known to be chronically painful has
been made on the basis of objective findings; and
7.2.3.2 All
reasonable non-surgical treatment has been exhausted; and
7.2.3.3 Pre-surgical
psychiatric or psychological evaluation has been performed and has demonstrated
motivation and long-term commitment without issues of secondary gain;
7.2.3.4 There
is no evidence of addictive behavior. (Tolerance and dependence to narcotic
analgesics are not addictive behaviors and do not preclude implantation.); and
7.2.3.5 A
successful trial. A screening test is considered successful if the patient (a)
experiences a 50% decrease in pain, which may be confirmed by VAS.
7.2.3.6 A mandatory second opinion is required to
confirm the rationale for the procedure in non malignant pain.
7.3 FACET
RHIZOTOMY
7.3.1 Description
– A procedure designed to denervate the facet joint by ablating the
periarticular facet nerve branches. There is good evidence to support this procedure
for the cervical spine and some evidence in lumbar spine.
7.3.2 Indications
– Pain of facet origin, unresponsive to active and/or passive therapy. All
patients must have a successful response to diagnostic medial nerve branch
blocks. A successful response is considered to be a 50% or greater relief of
pain for the length of time appropriate to the local anesthetic.
7.3.3 Operative Treatment – Percutaneous
radio-frequency rhizotomy is the procedure of choice over alcohol, phenol, or
cryoablation. Position of the probe using fluoroscopic guidance is required.
Successful management of chronic pain
conditions results in fewer relapses requiring intense medical care. Failure to
address long-term management as part of the overall treatment program may lead
to higher costs and greater dependence on the health care system.
Maintenance care in CRPS and CPD requires a
close working relationship between the carrier, the providers, and the patient.
Providers and patients have an obligation to design a cost-effective, medically
appropriate program that is predictable and allows the carrier to set aside
appropriate reserves. Carriers and adjusters have an obligation to assure that
medical providers can design medically appropriate programs. A designated
primary physician for maintenance team management is recommended.
Maintenance care will be based on principles
of patient self-management. When developing a maintenance plan of care, the
patient, physician and insurer should attempt to meet the following goals:
8.1 Maximal
independence will be achieved through the use of home exercise programs or
exercise programs requiring special facilities (e.g., pool, health club) and
educational programs; b. modalities will emphasize self-management and
self-applied treatment;
8.2 Management
of pain or injury exacerbations will emphasize initiation of active therapy
techniques and may require anesthetic injection blocks.
8.3 Dependence
on treatment provided by practitioners other than the authorized treating
physician will be minimized;
8.4 Periodic
reassessment of the patient’s condition will occur as appropriate.
8.5 Patients
will understand that failure to comply with the elements of the self-management
program or
therapeutic plan of care may affect
consideration of other interventions.
The following are Specific Maintenance Interventions and Parameters:
8.5.1 HOME
EXERCISE PROGRAMS AND EXERCISE EQUIPMENT Most patients have the ability to participate in a home exercise program after completion of a supervised exercise rehabilitation program. Programs should incorporate an exercise prescription including the continuation of an age-adjusted and diagnosis-specific program for aerobic conditioning, flexibility, stabilization, and strength. Some patients may benefit from the purchase or rental of equipment to maintain a home exercise program. Determination for the need of home equipment should be based on medical necessity, compliance with an independent exercise program, and reasonable cost. Before the purchase or long-term rental of equipment, the patient should be able to demonstrate the proper use and effectiveness of the equipment. Effectiveness of equipment should be evaluated on its ability to improve or maintain functional areas related to activities of daily living or work activity. Occasionally, compliance evaluations may be made through a 4-week membership at a facility offering similar equipment. Home exercise programs are most effective when done 3 to 5 times a week.
8.5.2 EXERCISE PROGRAMS
REQUIRING SPECIAL FACILITIES Some patients may have higher compliance with
an independent exercise program at a health club versus participation in a home
program. All exercise programs completed through a health club facility should
focus on the same parameters of an age-adjusted and diagnosis-specific program
for aerobic conditioning, flexibility, stabilization, and strength. Selection
of health club facilities should be limited to those able to track attendance
and utilization, and provide records available for physician and insurer
review. Prior to purchasing a membership, a therapist and/or exercise
specialist who has treated the patient may visit the facility with the patient
to assure proper use of the equipment. Frequency: 2 to 3 times per week.
Optimal duration: 1 to 3 months. Maximum maintenance duration: 3 months. Continuation
beyond 3 months should be based on functional benefit and patient compliance.
Health club membership should not extend beyond 3 months if attendance drops
below 2 times
per
week on a regular basis.
8.5.3 PATIENT EDUCATION
MANAGEMENT Educational classes, sessions, or programs may be necessary to
reinforce self-management techniques. This may be performed as formal or
informal programs, either group or individual.
Maintenance
duration: 2 to 6 educational sessions during one 12-month period.
8.5.4 PSYCHOLOGICAL MANAGEMENT
An ideal maintenance program will emphasize management options implemented
in the following order: (a) individual self-management (pain control,
relaxation and stress management, etc.), (b) group counseling, (c) individual
counseling, by a psychologist or psychiatrist, and (d) in-patient treatment.
Aggravation of the injury may require psychological treatment to restore the
patient to baseline.
Maintenance
duration: 6 to 10 visits during one 12-month period.
8.5.5 NON-NARCOTIC MEDICATION
MANAGEMENT In some cases, self-management of pain and injury exacerbations
can be handled with medications, such as those listed in the Medication
section. Physicians must follow patients who are on any chronic medication or
prescription regimen for efficacy and side effects. Laboratory or other testing
may be appropriate to monitor medication effects on organ function.
Maintenance
duration: Usually, four medication reviews within a 12-month period.
Frequency depends on the medications prescribed. Laboratory and other
monitoring as
appropriate.
8.5.6 NARCOTIC MEDICATION
MANAGEMENT As compared with other pain syndromes, there may be a role for
chronic augmentation of the maintenance program with narcotic medications In selected cases, scheduled medications may
prove to be the most cost effective means of insuring the highest function and
quality of life. A patient should have met the criteria in the opioids section
of these guidelines before beginning maintenance narcotics. Laboratory or other
testing may be appropriate to monitor medication effects on organ function. The
following management is suggested for maintenance narcotics:
8.5.6.1 A
narcotic medication regimen should be defined, which may increase or decrease
over time. Dosages will need to be adjusted based on side effects of the
medication and objective function of the patient. A patient may frequently be
maintained on additional nonnarcotic medications to control side effects,
treat mood disorders, or control neuropathic pain; however, only one
long-acting narcotic and one short acting narcotic for rescue use should be
prescribed in most cases.
8.5.6.2 All
patients on chronic narcotic medication dosages need to sign an appropriate
narcotic contract with their physician for prescribing the narcotics.
8.5.6.3 The
patient must understand that continuation of the medication is contingent on
their cooperation with the maintenance program. Use of non-prescribed drugs may
result in tapering of the medication. The clinician may order random drug
testing when deemed appropriate to monitor medication compliance.
8.5.6.4 Patients
on chronic narcotic medication dosages must receive them through one
prescribing physician or physician group.
Maintenance: Up to 12 visits within a
12-month period to review the narcotic plan.
Laboratory
and other monitoring as appropriate.
8.5.7 THERAPY
MANAGEMENT Some treatment may be helpful on a continued basis during
maintenance care if the therapy maintains objective function and decreases
medication use. Aggravation the injury may require intensive treatment to get
the patient back to baseline. In those cases, treatments and time frame
parameters listed in the Active and Passive Therapy sections apply.
Active Therapy, Acupuncture, and
Manipulation maintenance duration: 10 visits in a 12-month period.
8.5.8 INJECTION
THERAPY
8.5.8.1 Sympathetic
Blocks - These injections are considered appropriate if they maintain or
increase function. Maintenance blocks are usually combined with and enhanced by
the appropriate neuropharmacological medication(s) and other care. It is
anticipated that the frequency of the maintenance blocks may increase in the
cold winter months or with stress.
Maintenance duration: Not to exceed 6 to 8
blocks in a 12-month period for a single. Increased frequency may need to be
considered for multiple extremity involvement or for acute recurrences of pain
and symptoms. For treatment of acute exacerbations, consider 2 to 6 blocks with
a short time interval between blocks.
8.5.8.2 Trigger
Point Injections -These injections may occasionally be necessary to maintain
function in those with myofascial problems.
Maintenance duration: Not more than 4 injections per session not to exceed 6
sessions
per 12-month period.
8.5.8.3 Epidural
and Selective Nerve Root Injections - Patients who have experienced functional
benefits from these injections in the past may require injection for
exacerbations of the condition.
Maintenance duration: 6 treatments per
12-month period (a treatment may involve injection at one or two levels.)
8.5.9 PURCHASE
OR RENTAL OF DURABLE MEDICAL EQUIPMENT It is recognized that some patients
may require ongoing use of self-directed modalities for the purpose of
maintaining function and/or analgesic effect. Purchase or rental of modality
based equipment should be done only if the assessment by the physician and/or
therapist has determined the effectiveness, compliance, and improved or
maintained function by its application. It is generally felt that large expense
purchases such as spas, whirlpools, and special mattresses are not necessary to
maintain function beyond the areas listed above.
Maintenance duration: Not
to exceed 3 months for rental equipment. Purchase if effective.
PART C CUMULATIVE TRAUMA DISORDER MEDICAL
TREATMENT GUIDELINES
Pursuant to 19 Del.C. §2322C, health
care practice guidelines have been adopted and recommended by the Health Care
Advisory Panel to guide utilization of health care treatments in workers'
compensation including, but not limited to, care provided for the treatment of
employees by or under the supervision of a licensed health care provider,
prescription drug utilization, inpatient hospitalization and length of stay,
diagnostic testing, physical therapy, chiropractic care and palliative care.
The health care practice guidelines apply to all treatments provided after the
effective date of the regulation adopted by the Department of Labor, May 23, 2008,
and regardless of the date of injury. The guidelines are, to the extent
permitted by the most current medical science or applicable science, based on
well-documented scientific research concerning efficacious treatment for
injuries and occupational disease. To the extent that well-documented
scientific research regarding the above is not available at the time of
adoption of the guidelines, or is not available at the time of any revision to
the guidelines, the guidelines have been and will be based upon the best
available information concerning national consensus regarding best health care
practices in the relevant health care community.
The
guidelines, to the extent practical and consistent with the Act, address
treatment of those physical conditions which occur with the greatest frequency,
or which require the most expensive treatments, for work-related injuries based
upon currently available Delaware
data.
Services
rendered by any health care provider certified pursuant to 19 Del.C. §2322D(a)
to provide treatment or services for injured employees shall be presumed, in
the absence of contrary evidence, to be reasonable and necessary if such
treatment and/or services conform to the most current version of the Delaware
health care practice guidelines.
Services
rendered outside the Guidelines and/or variation in treatment recommendations
from the Guidelines may represent acceptable medical care, be considered
reasonable and necessary treatment and, therefore, determined to be
compensable, absent evidence to the contrary, and may be payable in accordance
with the Fee Schedule and Statute, accordingly.
Services
provided by any health care provider that is not certified pursuant to 19 Del.C.
§2322D(a) shall not be presumed reasonable and necessary unless such services
are pre-authorized by the employer or insurance carrier, subject to the
exception set forth in 19 Del.C. §2322D(b).
Treatment
of conditions unrelated to the injuries sustained in an industrial accident may
be denied as unauthorized if the treatment is directed toward the
non-industrial condition, unless the treatment of the unrelated injury is
rendered necessary as a result of the industrial accident.
The
Health Care Advisory Panel and Department of Labor recognized that acceptable
medical practice may include deviations from these Guidelines, as individual
cases dictate. Therefore, these Guidelines are not relevant as evidence of a
provider's legal standard of professional care.
In accordance with the requirements of the
Act, the development of the health care guidelines has been directed by a
predominantly medical or other health professional panel, with recommendations
then made to the Health Care Advisory Panel.
The
principles summarized in this section are key to the intended implementation of
all Division of Workers’ Compensation guidelines and critical to the reader’s
application of the guidelines in this document.
2.1 EDUCATION
of the patient and family, as well as the employer, insurer, policy makers and
the community should be emphasized in the treatment of CTD and disability.
Practitioners may develop and implement an effective strategy and skills to
educate patients, employers, insurance systems, policy makers and the community
as a whole.
2.2 TREATMENT
PARAMATER Time frames for specific interventions commence once treatments
have been initiated, not on the date of injury. Obviously, duration will be
impacted by patient compliance, comorbities and availability of services.
Clinical judgment may substantiate the need to modify the total number of
visits discussed in this document. The majority of injured workers with
Cumulative Trauma Disorders often will achieve resolution of their condition
within 6 to 36 visits (Guide To Physical Therapy Practice – Second
Edition). It is anticipated that most
injured workers will not require the maximum number of visits described in
these guidelines. They are designed to be
a ceiling and care extending beyond the maximum allowed visits may warrant utilization
review.
2.3 ACTIVE
INTERVENTIONS emphasizing patient responsibility, such as therapeutic
exercise and/or functional treatment, are generally emphasized over passive
modalities, especially as treatment progresses. Generally, passive
interventions are viewed as a means to facilitate progress in an active
rehabilitation program with concomitant attainment of objective functional
gains. All rehabilitation programs must incorporate “Active Interventions” no
later than three weeks after the onset of treatment. Reimbursement for passive
modalities only after the first three weeks of treatment without clear evidence
of Active Interventions will require supportive documentation.
2.4 ACTIVE
THERAPEUTIC EXERCISE PROGRAM Exercise program goals should incorporate
patient strength, endurance, flexibility, coordination, and education. This
includes functional application in vocational or community settings
2.5 POSITIVE
PATIENT RESPONSE results are defined primarily as functional gains that can
be objectively measured. Objective functional gains include, but are not
limited to, positional tolerances, range of motion, strength, endurance,
activities of daily living, cognition, psychological behavior, and
efficiency/velocity measures that can be quantified. Subjective reports of pain
and function should be considered and given relative weight when the pain has
anatomic and physiologic correlation. Anatomic correlation must be based on
objective findings.
2.6 RE-EVALUATE
TREATMENT EVERY 3 TO 4 WEEKS If a given treatment or modality is not
producing positive results within 3 to 4 weeks, the treatment should be either
modified or discontinued. Reconsideration of diagnosis should also occur in the
event of poor response to a seemingly rational intervention.
2.7 SURGICAL
INTERVENTIONS Surgery should be contemplated within the context of expected
functional outcome and not purely for the purpose of pain relief. All operative
interventions must be based upon positive correlation of clinical findings,
clinical course, and diagnostic tests. A comprehensive assimilation of these
factors must lead to a specific diagnosis with positive identification of
pathologic conditions.
2.8 SIX-MONTH
TIME FRAME The prognosis drops precipitously for returning an injured
worker to work once he/she has been temporarily totally disabled for more than
six months. The emphasis within these guidelines is to move patients along a
continuum of care and return-to-work within a six-month time frame, whenever
possible. It is important to note that time frames may not be pertinent to
injuries that do not involve work-time loss or are not occupationally related.
2.9 RETURN-TO-WORK
is therapeutic, assuming the work is not likely to aggravate the basic
problem or increase long-term pain. The practitioner must provide specific
physical limitations per the Physician’s Report form. The following physical
limitations should be considered and modified as recommended: lifting, pushing,
pulling, crouching, walking, using stairs, bending at the waist, awkward and/or
sustained postures, tolerance for sitting or standing, hot and cold
environments, data entry and other repetitive motion tasks, sustained grip,
tool usage and vibration factors. Even if there is residual chronic pain, return-to-work
is not necessarily contraindicated.
The practitioner should understand all of
the physical demands of the patient’s job position before returning the patient
to full duty and should receive clarification of the patient’s job duties.
2.10 DELAYED
RECOVERY Strongly consider a psychological evaluation, if not previously
provided, as well as initiating interdisciplinary rehabilitation treatment and
vocational goal setting, for those patients who are failing to make expected
progress 6 to 12 weeks after an injury. The Division recognizes that 3 to 10%
of all industrially injured patients will not recover within the time lines
outlined in this document despite optimal care. Such individuals may require
treatments beyond the limits discussed within this document, but such treatment
will require clear documentation by the authorized treating practitioner
focusing on objective functional gains afforded by further treatment and impact
upon prognosis.
2.11 GUIDELINE
RECOMMENDATIONS AND INCLUSION OF MEDICAL EVIDENCE are recommendations based
on available evidence and/or consensus recommendations. When possible,
guideline recommendations will note the level of evidence supporting the
treatment recommendation.
All recommendations in the guideline are
considered to represent reasonable care in appropriately selected cases,
regardless of the level of evidence or consensus statement attached to it.
Those procedures considered inappropriate, unreasonable, or unnecessary are
designated in the guideline as being “not recommended.”
The remainder of this document should be
interpreted within the parameters of these guideline principles that may lead
to more optimal medical and functional outcomes for injured workers.
Cumulative Trauma Disorders (CTDs) of the
upper extremity comprise a heterogeneous group of diagnoses which include
numerous specific clinical entities, including disorders of the muscles,
tendons and tendon sheaths, nerve entrapment syndromes, joint disorders, and
neurovascular disorders.
The terms “cumulative trauma disorder”,
“repetitive motion syndrome”, “repetitive strain injury” and
other similar nomenclatures are umbrella terms that are not acceptable
diagnoses. The health care provider must provide specific diagnoses in order to
appropriately educate, evaluate, and treat the patient. Examples include
DeQuervain’s tendonitis, cubital tunnel syndrome, lateral/medial epicondylitis,
olecranon bursitis, and hand-arm vibration syndrome. Many patients present with
more than one diagnosis, which requires thorough upper extremity and cervical
evaluation by the health care provider. Furthermore, there must be a causal
relationship between work activities and the diagnosis (see Initial Diagnostic
Procedures). The mere presence of a diagnosis that may be associated with
cumulative trauma does not presume work-relatedness unless the appropriate work
exposure is present.
Mechanisms of injury for the development of
CTDs remain controversial. Posture, repetition, force, vibration, cold
exposure, and combinations thereof are postulated and generally accepted as
risk factors for the development of CTDs. Evaluation of a CTD requires an
integrated approach that incorporates ergonomics, clinical assessment, and psychosocial
evaluation on a case-by-case basis.
History and physical examination (Hx &
PE) are generally accepted, well-established and widely used procedures which
establish the foundation/basis for and dictate all other diagnostic and
therapeutic procedures. When findings of clinical evaluations and those of
other diagnostic procedures do not complement each other, the objective
clinical findings should have preference.
4.1 HISTORY Should inquire about the
following issues, where relevant, and document pertinent positives and
negatives where appropriate. In evaluating potential CTDs, the following
actions should be taken:
4.1.1 Description of Symptoms:
4.1.1.1 Onset: date of onset, sudden vs.
gradual;
4.1.1.2 Nature of Symptoms:
pain, numbness, weakness, swelling, stiffness, temperature change, color
change;
4.1.1.3 Intensity: pain scale (0 = no pain,
and 10 = worst imaginable pain) may be used.
4.1.1.4 Location and Radiation: use of a
pain diagram is encouraged for characterizing sensory symptoms; use
comprehensive diagrams and do not use limited diagrams depicting only the hand
or arm, as it is important to solicit the reporting of more proximal symptoms;
4.1.1.5 Provocative and Alleviating Factors
(occupational and non-occupational): Attempt to identify the specific physical
factors that are aggravating or alleviating the problem;
4.1.1.6 Sleep disturbances;
4.1.1.7 Other associated signs and symptoms
noted by the injured worker;
4.1.2 Identification
of Occupational Risk Factors: Job title alone is not sufficient
information. The clinician is responsible for documenting specific information
regarding repetition, force and other risk factors, as listed in the Risk
Factors Associated with Cumulative Trauma Table. A job site evaluation may be
required.
4.1.3 Demographics:
age, hand dominance, gender, etc.
4.1.4 Past Medical History and Review of
Systems:
4.1.4.1 Past injury/symptoms involving the
upper extremities, trunk and cervical spine;
4.1.4.2 Past work-related injury or
occupational disease;
4.1.4.3 Past personal injury or disease that
resulted in temporary or permanent job limitation;
4.1.4.4 Medical conditions associated with
CTD - A study of work-related upper extremity disorder patients showed a 30%
prevalence of co-existing disease. Medical conditions commonly occurring with
CTD include:
4.1.4.4.1 Pregnancy,
4.1.4.4.2 Arthropathies
including connective tissue disorders, rheumatoid arthritis, systemic lupus
erythematosus, gout, osteoarthritis and spondyloarthropathy,
4.1.4.4.3 Amyloidosis,
4.1.4.4.4 Hypothyroidism,
especially in older females,
4.1.4.4.5 Diabetes
mellitus, including family history or gestational diabetes,
4.1.4.4.6 Acromegaly,
4.1.4.4.7 Use
of corticosteroids.
4.1.5 Activities
of Daily Living (ADLs): ADLs include such activities as self care and
personal hygiene, communication, ambulation, attaining all normal living
postures, travel, non-specialized hand activities, sexual function, sleep, and
social and recreational activities. Specific movements in this category include
pinching or grasping keys/pens/other small objects, grasping telephone
receivers or cups or other similar-sized objects, and opening jars. The quality
of these activities is judged by their independence, appropriateness, and
effectiveness. Assess not simply the number of restricted activities but the
overall degree of restriction or combination of restrictions.
4.1.6 other
avocational activities that might contribute to or be impacted by CTD
development. Activities such as hand-operated video games,
crocheting/needlepoint, home computer operation, golf, tennis, and gardening
are included in this category.
4.1.7 Social
History: Exercise habits, alcohol consumption, and psychosocial factors.
4.2 PHYSICAL
EXAMINATION The evaluation of any upper extremity complaint should begin at
the neck and upper back and then proceed down to the fingers and include the
contralateral region. It should include evaluation of vascular and neurologic
status, and describe any dystrophic changes or variation in skin color or
turgor.
Table 1: Physical Examination Findings
Reference Table
TITLE
19 LABOR
DELAWARE
ADMINISTRATIVE CODE
|
DIAGNOSIS
|
SYMPTOMS
|
SIGNS
|
|
DeQuervain’s Tenosynovitis
|
Pain and swelling in the
anatomical snuffbox; pain radiating into the hand and forearm; pain worsened
by thumb abduction and/or extension.
|
Pain worsened by active thumb
abduction and/or extension; crepitus along the radial forearm; positive
Finkelstein’s.
|
|
Extensor Tendinous Disorders
|
Pain localized to the
affected tendon(s); pain worsened by active and/or resisted wrist or finger
extension.
|
Swelling along the dorsal
aspects of the hand/wrist/ forearm, and pain with active and/or resisted
wrist/ digit extension, or creaking/crepitus with wrist extension.
|
|
Flexor Tendinous Disorders
|
Pain localized to the
affected tendons; pain in the affected tendons associated with wrist flexion
and ulnar deviation, especially against resistance.
|
Pain with wrist/digit flexion
and ulnar deviation, or crepitus with active motion of the flexor tendons.
|
|
Lateral Epicondylitis
|
Lateral elbow pain
exacerbated by repetitive wrist motions; pain emanating from the lateral
aspect of the elbow.
|
Pain localized to lateral
epicondyle with resisted wrist extension and/or resisted supination.
|
|
Medial Epicondylitis
|
Pain emanating from the
medial elbow; mild grip weakness; medial elbow pain exacerbated by repetitive
wrist motions.
|
Pain localized to the medial
epicondyle with resisted wrist flexion and resisted pronation.
|
|
Cubital tunnel syndrome
|
Activity-related
pain/paresthesias involving the 4th and 5th fingers coupled with pain in the
medial aspect of the elbow; pain/ paresthesias worse at night; decreased
sensation of the 5th finger and ulnar half of the ring finger (including
dorsum 5th finger); progressive inability to separate fingers; loss of power
grip and dexterity; atrophy/weakness of the ulnar intrinsic hand muscles
(late sign).
|
Diminished sensation of the
fifth and ulnar half of the ring fingers; elbow flexion/ulnar compression
test; Tinels’ sign between olecranon process and medial epicondyle; Later
stages manifested by intrinsic atrophy and ulnar innervated intrinsic
weakness. Specific physical signs include clawing of the ulnar 2 digits
(Benediction posture), ulnar drift of the 5th finger (Wartenberg’s sign), or
flexion at the thumb IP joint during pinch (Froment’s sign).
|
|
Hand-Arm Vibration Syndrome
|
Pain/paresthesias in the
digits; blanching of the digits; cold intolerance; tenderness/swelling of the
digits/hand/forearm; muscle weakness of the hand; joint pains in
hand/wrist/elbow/neck/ shoulders; trophic skin changes and cyanotic color in
hand/digits.
|
Sensory deficits in the
digits/hand; blanching of digits; swelling of the digits/hand/forearm; muscle
weakness of the hand; arthropathy at the hand/wrist/elbow; trophic skin
changes and cyanotic color in hand/ digits.
|
TITLE
19 LABOR
DELAWARE
ADMINISTRATIVE CODE
|
Guyon Canal (Tunnel) Syndrome
|
Numbness/tingling in ulnar
nerve distribution distal to wrist.
|
Positive Tinel’s at hook of
hamate. Numbness or paresthesias of the palmar surface of the ring and small
fingers. Later stages may affect ulnar innervated intrinsic muscle strength.
|
|
Pronator Syndrome
|
Pain/numbness/tingling in
median nerve distribution distal to elbow.
|
Tingling in median nerve
distribution on resisted pronation with elbow flexed at 90o Tenderness or
Tinel’s at the proximal edge of the pronator teres muscle over the median
nerve.
|
|
Radial Tunnel Syndrome
|
Numbness/tingling or pain in
the lateral posterior forearm.
|
Tenderness over the radial
nerve near the proximal edge of the supinator muscle. Rarely, paresthesias in
the radial nerve distribution or weakness of thumb or finger extension.
|
4.3 PAIN BEHAVIOR EVALUATION
4.3.1 Evaluate
the patient's overall pain behavior. The behavior should be consistent with the
current pain levels reported by the patient.
4.3.2 Use
a measurement tool to quantify and/or qualify pain. Reference the pain scale
(0-10) with the worst pain imaginable being the top end of the scale (10)
and/or other pain scales such as the Visual Analog Scale, Pain Drawing, Neck
Disability Index, or McGill Pain Questionnaire.
4.4 RISK
FACTORS A critical review of epidemiologic literature identifies a number
of physical exposures associated with CTDs. Physical exposures considered risk
factors include: repetition, force, vibration, pinching and gripping, and cold
environment. When workers are exposed to several risk factors simultaneously,
there is an increased likelihood of a CTD. Not all risk factors have been
extensively studied. Exposure to cold environment, for example, was not
examined independently; however, there is good evidence that combined with
other risk factors, cold environment increases the likelihood of a CTD. The
table at the end of this section entitled, "Risk Factors Associated
CTDs," summarizes the results of currently available literature.
No single epidemiologic study will fulfill
all criteria for causality. The clinician must recognize that currently
available epidemiologic data is based on population results, and that
individual variability lies outside the scope of these studies. Many published
studies are limited in design and methodology, and, thus, preclude conclusive
results. Most studies' limitations tend to attenuate, rather than inflate,
associations between workplace exposures and CTDs.
Many specific disorders, such as ulnar
neuropathy (at the elbow and wrist) and pronator teres syndrome, have not been
studied sufficiently to formulate evidence statements regarding causality.
Based on the present understanding of mechanism of injury and utilizing the
rationale of analogy, it is generally accepted that these disorders are similar
to other CTDs at the elbow and wrist and are susceptible to the same risk
factors. No studies examined the relationship between the development of
ganglion cysts and work activities; however, work activities may aggravate
existing ganglion cysts. It is generally accepted that keyboarding less than
four hours per day is unlikely to be associated with a CTD when no other risk
factors are present. It remains unclear how computer mouse use affects CTDs.
The posture involved in mouse use should always be evaluated when assessing
risk factors.
Studies measured posture, repetition, and
force in variable manners. In general, jobs that require less than 50% of
maximum voluntary contractile strength for the individual are not considered
“high force.”
Likewise, jobs with
wrist postures less than or equal to 25o flexion or extension, or ulnar deviation less
than or equal to 10o are not likely to cause posture problems.
These guidelines are based on current epidemiologic knowledge. As with any scientific
work, the
guidelines are expected to change with advancing
knowledge. The clinician should remain flexible and consider new information
revealed in future studies.
Table 2: Risk Factors
Associated with Cumulative Trauma
TITLE 19 LABOR
DELAWARE
ADMINISTRATIVE CODE
|
Diagnosis
|
Strong evidence
|
Good evidence
|
Some evidence
|
Insufficient or
conflicting evidence
|
|
Elbow Musculoskeletal
Disorders (Epicondylitis)
|
Combination high force and
high repetition (Exposures were based on EMG data, observation or video
analysis of job tasks, or categorization by job title. Observed movements
include repeated extension, flexion, pronation and supination. Repetition
work cycles less than 30 sec. or greater than 50% of cycle time performing
same task, and number of items assembled in one hour).
|
High force alone
|
|
Repetition alone, extreme
wrist posture.
|
|
Wrist Tendonitis, including
DeQuervain's Tenosynovitis
|
Combination of risk factors:
High repetition, forceful hand/wrist exertions, extreme wrist postures
(Assessed by direct observation, EMG, and video analysis. One study measured
time spent in deviated wrist posture).
|
Repetition, (as previously
defined), not including keyboarding or force independently
|
Posture
|
|
|
Trigger Finger
|
|
|
Forceful grip (Holding tools,
knives. Assessed by direct observation and video analysis).
|
|
5.1 ELECTRODIAGNOSTIC (EDX) STUDIES
5.1.1 Electrodiagnostic
(EDX) studies are well-established and widely accepted for evaluation of
patients suspected of having peripheral nerve pathology. Studies may confirm
the diagnosis or direct the examiner to alternative disorders. Studies may
require clinical correlation due to the occurrence of false positive and false
negative results. Symptoms of peripheral nerve pathology may occur with normal
EDX studies, especially early in the clinical course. Findings include
fibrillations, fasciculations, neurogenic recruitment, and polyphasic units
(reinnervation).
5.1.2 To
assure accurate testing, temperature should be maintained at 30-34C preferably
recorded from the hand/digits. For temperature below 30C the hand should be
warmed.
5.1.3 All studies must include normative values for
their laboratories.
5.2 IMAGING STUDIES
5.2.1 Radiographic
Imaging: Not generally required for most CTD diagnoses. However, it may be
necessary to rule out other pathology in the cervical spine, shoulder, elbow,
wrist, or hand. Wrist and elbow radiographs would detect degenerative joint
disease, particularly scapholunate dissociation and thumb carpometacarpal
abnormalities which occasionally occur with CTD.
5.2.2 MRI: May show increased T2-weighted
signal intensity of the common extensor tendon in lateral epicondylitis, but
this finding has commonly been found in the asymptomatic contralateral elbow
and may not be sufficiently specific to warrant the use of MRI as a diagnostic
test for epicondylitis. Its routine use for CTD is not recommended.
5.3 ADJUNCTIVE TESTING
5.3.1 Personality/Psychological/Psychosocial
Evaluations: are generally accepted and well-established diagnostic
procedures with selective use in the CTD population, but have more widespread
use in sub-acute and chronic pain populations.
Diagnostic testing procedures may be useful
for patients with symptoms of depression, delayed recovery, chronic pain,
recurrent painful conditions, disability problems, and for pre-operative
evaluation as well as a possible predictive value for post-operative response.
Psychological testing should provide differentiation between pre-existing
depression versus injury-caused depression, as well as post-traumatic stress
disorder.
Formal psychological or psychosocial
evaluation should be performed on patients not making expected progress within
6-12 weeks following injury and whose subjective symptoms do not correlate with
objective signs and tests. In addition to the customary initial exam, the
evaluation of the injured worker should specifically address the following
areas:
5.3.1.1 Employment history;
5.3.1.2 Interpersonal relationships — both
social and work;
5.3.1.3 Leisure activities;
5.3.1.4 Current perception of the medical
system;
5.3.1.5 Results of current treatment;
5.3.1.6 Perceived locus of control; and
5.3.1.7 Childhood history, including abuse and family
history of disability. Results should provide clinicians with a better
understanding of the patient, thus allowing for more effective rehabilitation.
The evaluation will determine the need for further psychosocial interventions,
and in those cases, a Diagnostic Statistical Manual for Mental Disorders (DSM)
diagnosis should be determined and documented. An individual with a Ph.D.,
PsyD, or Psychiatric MD/DO credentials may perform initial evaluations, which
are generally completed within one to two hours. When issues of chronic pain
are identified, the evaluation should be
more extensive and follow testing procedures as
outlined in the Division’s Chronic Pain Disorder Medical Treatment Guidelines.
• Frequency:
One time visit for evaluation. If psychometric testing is indicated as a portion
of the initial evaluation, time for such testing should not exceed an
additional two hours of professional time.
5.3.2 Laboratory
Tests: Generally accepted, well-established and widely used procedures.
Patients should be carefully screened at the initial exam for signs or symptoms
of diabetes, hypothyroidism, arthritis, and related inflammatory diseases. The
presence of concurrent disease does not negate work-relatedness of any specific
case. In one study of patients with cumulative trauma disorder other than
Carpal Tunnel Syndrome, seen by specialists, 3% of patients were diagnosed with
diabetes, 6% with hypothyroidism, and 9% with chronic inflammatory disease
including spondyloarthropathy, arthritis, and systemic lupus erythematosis. Up
to two thirds of the patients were not aware of their concurrent disease. When
a patient's history and physical examination suggest infection, metabolic or
endocrinologic disorders, tumorous conditions, systemic musculoskeletal
disorders (e.g., rheumatoid arthritis or ankylosing spondylitis), or problems
potentially related to medication (e.g., renal disease and nonsteroidal
anti-inflammatory medications), then laboratory tests, including, but not
limited to, the following can provide useful diagnostic information:
5.3.2.1 Serum
rheumatoid factor, Antinuclear Antigen (ANA), Human Leukocyte Antigen (HLA)B27
titre for rheumatoid work-up;
5.3.2.2 Thyroid
stimulating hormone (TSH) for hypothyroidism;
5.3.2.3 Fasting
glucose - recommended for obese men and women over 40 years of age, patients
with a history of family diabetes, those from high risk ethnic groups, and with
a previous history of impaired glucose tolerance. A fasting blood glucose
greater than 125mg/dl is diagnostic for diabetes. Urine dipstick positive for
glucose is a specific but not sensitive screening test. Quantitative urine
glucose is sensitive and specific in high risk populations;
5.3.2.4 Serum
protein electrophoresis;
5.3.2.5 Sedimentation
rate and C-Reactive Protein are nonspecific, but elevated in infection,
neoplastic conditions and rheumatoid arthritis;
5.3.2.6 Serum
calcium, phosphorus, uric acid, alkaline and acid phosphatase for metabolic,
endocrine and neo-plastic conditions;
5.3.2.7 complete
blood count (CBC), liver and kidney function profiles for metabolic or
endocrine disorders or for adverse effects of various medications;
5.3.2.8 Bacteriological
(microorganism) work-up for wound, blood and tissue. The Division recommends the
above diagnostic procedures be considered, at least initially, the
responsibility of the workers' compensation carrier to ensure that an accurate
diagnosis and treatment plan can be
established. Laboratory testing may be required periodically to monitor
patients on chronic medications.
5.3.3 Pinch
and Grip Strength Measurements: May be accepted as a diagnostic tool for
CTD. Strength is defined as the muscle force exerted by a muscle or group of
muscles to overcome a resistance under a specific set of circumstances. Pain,
the perception of pain secondary to abnormal sensory feedback, and/or the
presence of abnormal sensory feedback affecting the sensation of the power used
in grip/pinch may cause a decrease in the force exerted. When a bell-shaped
curve is present, these measures provide a method for quantifying strength that
can be used to follow a patient's progress and to assess response to therapy.
In the absence of a bell-shaped curve, clinical reassessment is indicated.
5.3.4 Quantitative
Sensory Testing (QST): May be used as a screening tool in clinical settings
pre-and post-operatively. Results of tests and measurements of sensory
integrity are integrated with the history and review of systems findings and
the results of other tests and measures. QST tests the entire sensory pathway,
limiting its ability to localize a deficit precisely. It depends on the
patient’s report of perception and may not be objective. Cutaneous conditions
may alter sensory thresholds.
5.3.4.1 Threshold
tests measure topognosis, the ability to exactly localize a cutaneous
sensation, and pallesthesia, the ability to detect mechanical sensation using
vibration discrimination testing (quickly adapting fibers); and/or
Semmes-Wienstein monofilament testing (slowly adapting fibers);
5.3.4.2 Density
Tests also measure topognosis and pallesthesia using static two-point
discrimination (slowly adapting fibers); and/or moving two-point discrimination
(quickly adapting fibers).
Before initiation of any therapeutic procedure, the
authorized treating provider, employer and insurer
must
consider these important issues in the care of the injured worker.
First, patients undergoing therapeutic procedure(s) should be released or
returned to modified or
restricted duty during their rehabilitation at the earliest appropriate time.
Refer to “Return-to-Work” in
this section for detailed information.
Second, cessation and/or review of treatment
modalities should be undertaken when no further significant subjective or
objective improvement in the patient’s condition is noted. If patients are not
responding within the recommended duration periods, alternative treatment
interventions, further diagnostic studies or consultations should be pursued.
Third, providers should provide and document
education to the patient. No treatment plan is complete without addressing
issues of individual and/or group patient education as a means of facilitating
self-management of symptoms.
Last, formal psychological or psychosocial
screening should be performed on patients not making expected progress within 6
to 12 weeks following injury and whose subjective symptoms do not correlate
with objective signs and tests.
In cases where a patient is unable to attend
an outpatient center, home therapy may be necessary. Home therapy may include
active and passive therapeutic procedures as well as other modalities to assist
in alleviating pain, swelling, and abnormal muscle tone. Home therapy is usually
of short duration and continues until the patient is able to tolerate coming to
an outpatient center.
The following procedures are listed in
alphabetical order.
6.1 ACUPUNCTURE
is an accepted and widely used procedure for the relief of pain and inflammation. The exact mode of action is only partially understood. Western medicine studies suggest that acupuncture stimulates the nervous system at the level of the brain, promotes deep relaxation, and affects the release of neurotransmitters. Acupuncture is commonly used as an alternative or in addition to traditional Western pharmaceuticals. While it is commonly used when pain medication is reduced or not tolerated, it may be used as an adjunct to physical rehabilitation and/or surgical intervention to hasten the return of functional activity. Acupuncture should be performed by MD, DO, DC with appropriate training; or a licensed acupuncturist.
6.1.1 Acupuncture:
is the insertion and removal of filiform needles to stimulate acupoints
(acupuncture points). Needles may be inserted, manipulated and retained for a
period of time. Acupuncture can be used to reduce pain and inflammation, and to
increase blood flow to an area and increase range of motion. Indications
include joint pain, joint stiffness, soft tissue pain and inflammation,
paresthesia, post-surgical pain relief, muscle spasm, and scar tissue pain.
·
Time to produce
effect: 3 to 6 treatments
·
Frequency: 1 to 3
times per week
·
Course duration:
14 treatments
6.1.2 Acupuncture
with Electrical Stimulation: is the use of electrical current
(micro-amperage or milli-amperage) on the needles at the acupuncture site. It
is used to increase effectiveness of the needles by continuous stimulation of
the acupoint. Physiological effects (depending on location and settings) can
include endorphin release for pain relief, reduction of inflammation, increased
blood circulation, analgesia through interruption of pain stimulus, and muscle
relaxation.
It is indicated to treat chronic pain
conditions, radiating pain along a nerve pathway, muscle spasm, inflammation,
scar tissue pain, and pain located in multiple sites.
·
Time to produce
effect: 3 to 6 treatments
·
Frequency: 1 to 3
times per week
·
Course duration:
14 treatments
6.1.3 Other
Acupuncture Modalities: Acupuncture treatment is based on individual
patient needs and therefore treatment may include a combination of procedures
to enhance treatment effect. Other procedures may include the use of heat, soft
tissue manipulation/massage, and exercise.
·
Time to produce
effect: 3 to 6 treatments
·
Frequency: 1 to 3
times per week
• Course duration: 14 treatments Any of the
above acupuncture treatments may extend longer if objective functional gains
can be documented or when symptomatic benefits facilitate progression in the
patient’s treatment
program. Treatment beyond 14 sessions (1
course) may be documented with respect to need and ability to facilitate
positive symptomatic or functional gains.
6.2 BIOFEEDBACK
is a form of behavioral medicine that helps patients learn self-awareness
and self-regulation skills for the purpose of gaining greater control of their
physiology, such as muscle activity, brain waves, and measures of autonomic
nervous system activity. Electronic instrumentation is used to monitor the
targeted physiology and then displayed or fed back to the patient visually,
auditorially, or tactilely, with coaching by a biofeedback specialist.
Biofeedback is provided by clinicians certified in biofeedback and/or who have
documented specialized education, advanced training, or direct or supervised
experience qualifying them to provide the specialized treatment needed (e.g.,
surface EMG, EEG, or other).
Treatment is individualized to the patient’s
work-related diagnosis and needs. Home practice of skills is required for
mastery and may be facilitated by the use of home training tapes. The ultimate
goal in biofeedback treatment is normalizing the physiology to the pre-injury
status to the extent possible and involves transfer of learned skills to the
workplace and daily life. Candidates for biofeedback therapy or training must
be motivated to learn and practice biofeedback and self-regulation techniques.
Indications for biofeedback include
individuals who are suffering from musculoskeletal injury where muscle
dysfunction or other physiological indicators of excessive or prolonged stress
response affects and/or delays recovery. Other applications include training to
improve self-management of emotional stress/pain responses such as anxiety,
depression, anger, sleep disturbance, and other central and autonomic nervous
system imbalances. Biofeedback is often utilized along with other treatment
modalities.
·
Time to produce
effect: 3 to 4 sessions
·
Frequency: 1 to 2
times per week
·
Maximum duration:
10 to 12 sessions. Treatment beyond 12 sessions must be documented with respect
to need, expectation, and ability to facilitate positive symptomatic or
functional gains.
6.3 INJECTIONS
– THERAPEUTIC are generally accepted, well-established procedures that may
play a significant role in the treatment of patients with upper extremity pain
or pathology. Therapeutic injections involve the delivery of anesthetic and/or
anti-inflammatory medications to the painful structure. Therapeutic injections
have many potential benefits. Ideally, a therapeutic injection will: (a) reduce
inflammation in a specific target area; (b) relieve secondary muscle spasm; and
(c) diminish pain and support therapy directed to functional recovery.
Diagnostic and therapeutic injections should be used early and selectively to
establish a diagnosis and support rehabilitation. If injections are overused or
used outside the context of a monitored rehabilitation program, they may be of
significantly less value.
6.3.1 Steroid
Injections: may provide both diagnostic and therapeutic value in treating a
variety of upper extremity cumulative trauma disorders. These include
neuropathies, tendonitis or bursitis about the elbow, wrist, or hand. In
contrast, there is no evidence to support their therapeutic use in other upper
extremity compressive neuropathies; however, it is a widely accepted procedure.
Steroid injections provide a potent anti-inflammatory
effect, which is usually short term in duration, lasting weeks or months.
Injections should always be used as an adjunctive treatment in the context of a
physical exercise and rehabilitation program.
For epicondylitis, there is good evidence
that although steroid injections with physical therapy may provide short-term
symptomatic relief, there is no benefit over placebo injections at 6 months. A
program of physical rehabilitation in combination with judicious use of
anti-inflammatory medications should be the core treatment for epicondylitis.
When performing tendon injections, the risk
of tendon rupture should be discussed with the patient and the need for
temporary restricted duty emphasized.
Contraindications:
General contraindications include local or systemic infection, bleeding
disorders, and allergy to medications used.
Local Steroid Injections:
·
Time to produce
effect: 3 days
·
Frequency:
monthly
·
Maximum duration:
3 injections
6.3.2 Trigger
Point Injections: are generally accepted, although used infrequently in
uncomplicated cases. They may, however, be used to relieve myofascial pain and
facilitate active therapy and stretching of the affected areas, and as an
adjunctive treatment in combination with other treatment modalities, such as
functional restoration programs, including stretching therapeutic exercise.
Trigger point injections should be utilized primarily for the purpose of
facilitating functional progress. The Division does not recommend their routine
use in the treatment of upper extremity injuries.
·
Time to produce
effect: Local anesthetic 30 minutes; 24 to 48 hours for no anesthesia.
·
Frequency:
Weekly. Suggest no more than 4 injection sites per session per week to avoid
significant post-injection soreness.
·
Maximum duration:
8 weeks. Occasional patients may require 2 to 4 repetitions of trigger point
injection series over a 1 to 2 year period.
6.3.3 Other
Injections: Some early evidence exists to support Autologous Blood Injection
may be used for medial/lateral epicondylitis. This can be repeated for a total
of 2-3 injections given roughly 6 weeks apart.
6.4 JOB
SITE ALTERATION Early evaluation and training of body mechanics and other
ergonomic factors are essential for every injured worker and should be done by
a qualified individual. In some cases, this requires a job site evaluation.
Some evidence supports alteration of the work site in the early treatment of
Cumulative Trauma Disorder. There is no single factor or combination of factors
that is proven to prevent or ameliorate CTD, but a combination of ergonomic and
psychosocial factors are generally considered to be important. Physical factors
that may be considered include use of force, repetition, awkward positions, upper
extremity vibration, cold environment, and contact pressure on the nerve.
Psychosocial factors to be considered include pacing, degree of control over
job duties, perception of job stress, and supervisory support.
The job analysis and modification should
include input from the employee, employer, and ergonomist or other professional
familiar with work place evaluation. The employee must be observed performing
all job functions in order for the job site analysis to be valid. Periodic
follow-up is recommended to evaluate effectiveness of the intervention and need
for additional ergonomic changes.
6.4.1 Ergonomic
changes: should be made to modify the hazards identified. In addition,
workers should be counseled to vary tasks throughout the day whenever possible.
OSHA suggests that workers who perform repetitive tasks, including keyboarding,
take 15-30 second breaks every 10 to 20 minutes, or 5-minute breaks every hour.
Mini breaks should include stretching exercises.
6.4.2 Interventions:
should consider engineering controls, e.g., mechanizing the task, changing the
tool used, or adjusting the job site; or administrative controls, e.g.,
adjusting the time an individual performs the task.
6.4.3 Seating
Description: The following description may aid in evaluating seated work
positions: The head should incline only slightly forward, and if a monitor is
used, there should be 18-24 inches of viewing distance with no glare. Arms
should rest naturally, with forearms parallel to the floor, elbows at the
sides, and wrists straight or minimally extended. The back must be properly
supported by a chair, which allows change in position and backrest adjustment.
There must be good knee and legroom, with the feet resting comfortably on the
floor or footrest. Tools should be within easy reach, and twisting or bending
should be avoided.
6.4.4 Job Hazard Checklist: The following
Table 4 is adopted from Washington
State’s job hazard
checklist, and may be used as a generally accepted guide for identifying job
duties which may pose ergonomic hazards. The fact that an ergonomic hazard
exists at a specific job, or is suggested in the table, does not establish a
causal relationship between the job and the individual with a musculoskeletal
injury. However, when an individual has a work-related injury and ergonomic
hazards exist that affect the injury, appropriate job modifications should be
made. Proper correction of hazards may prevent future injuries to others, as
well as aid in the recovery of the injured worker.
Table 4: Identifying Job Duties Which May
Pose Ergonomic Hazards
TITLE 19 LABOR
DELAWARE
ADMINISTRATIVE CODE
|
Type of Job Duty
|
Hours per Day
|
|
Pinching an unsupported
object(s) weighing 2 lbs or more per hand, or pinching with a force of 4 lbs
or more per hand (comparable to pinching a half a ream of paper): 1. Highly
repetitive motion 2. Palmar flexion greater than 30 degrees, dorsiflexion greater
than 45 degrees, or radial deviation greater than 30 degrees 3. No other risk
factors
|
More than 3 hours
total/day More than 4 hours total/day
|
|
Gripping an unsupported
object(s) weighing 10 lbs or more/hand, or gripping with a force of 10 lbs or
more/hand (comparable to clamping light duty automotive jumper cables onto a
battery): *Handles should be rounded and soft, with at least 1-2.5” in
diameter grips at least 5” long. 1. Highly repetitive motion 2. Palmar
flexion greater than 30 degrees, dorsiflexion greater than 45 degrees, or
radial deviation greater than 30 degrees 3. No other risk factors
|
More than 3 hours
total/day More than 4 hours total/day
|
|
Repetitive Motion (using the
same motion with little or no variation every few seconds) excluding keying
activities: 1. High, forceful exertions with the hands, with palmar flexion
greater than 30 degrees, dorsiflexion greater than 45 degrees, or radial deviation
greater than 30 degrees 2. No other risk factors
|
More than 2 hours
total/day More than 6 hours total/day
|
|
Intensive Keying: 1. Palmar
flexion greater than 30 degrees, dorsiflexion greater than 45 degrees, or
radial deviation greater than 30 degrees 2. No other risk factors
|
More than 4 hours
total/day More than 7 hours total/day
|
|
Repeated Impact: 1. Using the
hand (heel/base of palm) as a hammer more than once/minute
|
More than 2 hours
total/day
|
TITLE
19 LABOR
DELAWARE
ADMINISTRATIVE CODE
|
Vibration:
|
|
Two determinants of the
tolerability of segmental vibration of the hand are the
|
|
frequency and the
acceleration of the motion of the vibrating tool, with lower
|
|
frequencies being more poorly
tolerated at a given level of imposed acceleration,
|
|
expressed below in multiples
of the acceleration due to gravity (10m/sec./sec.).
|
More than 30 minutes
|
|
1. Frequency range 8-15 Hz
and acceleration 6 g
|
at a time
|
|
2. Frequency range 80 Hz and
acceleration 40 g
|
|
|
3. Frequency range 250 Hz and
acceleration 250 g
|
|
|
------------------------------------------------------
|
More than 4 hours at a
|
|
4. Frequency range 8-15 Hz
and acceleration 1.5 g
|
time
|
|
5. Frequency range 80 Hz and
acceleration 6 g
|
|
|
6. Frequency range 250 Hz and
acceleration 20 g
|
|
6.5 MEDICATIONS Medication
use in the treatment of CTD is appropriate for controlling acute and chronic
pain and inflammation. Use of medications will vary widely due to the spectrum
of injuries from simple strains to post-surgical analgesia. A thorough
medication history, including use of alternative and over the counter
medications, should be performed at the time of the initial visit and updated
periodically.
Acetaminophen is an effective and safe
initial analgesic. Nonsteroidal anti-inflammatory drugs (NSAIDs) are useful in
the treatment of inflammation, and for pain control. Pain is subjective in
nature and should be evaluated using a scale to rate effectiveness of the
analgesic in terms of functional gain. Other medications, including
antidepressants, may be useful in selected patients with chronic pain (Refer to
the Division’s Chronic Pain Guidelines). Narcotics are rarely indicated for
treatment of upper extremity CTDs, and they should be primarily reserved for
the treatment of acute severe pain for a limited time on a case-by-case basis.
Topical agents may be beneficial in the management of localized upper extremity
pain.
The following are listed in alphabetical
order:
6.5.1 Acetaminophen:
is an effective analgesic with antipyretic but not anti-inflammatory activity.
Acetaminophen is generally well tolerated, causes little or no gastrointestinal
irritation and is not associated with ulcer formation. Acetaminophen has been
associated with liver toxicity in doses over 10 gm/day or in chronic alcohol
use.
6.5.2 Minor
Tranquilizer/Muscle Relaxants: are appropriate for muscle spasm, mild pain
and sleep disorders.
6.5.3 Narcotics:
medications should be prescribed with strict time, quantity and duration
guidelines, and with definitive cessation parameters. Adverse effects include
respiratory depression, impaired alertness, and the development of physical and
psychological dependence.
• treatment.
6.5.4 Nonsteroidal
Anti-Inflammatory Drugs (NSAIDs)): are useful for pain and inflammation. In
mild cases, they may be the only drugs required for analgesia. There are
several classes of NSAIDs, and the response of the individual injured worker to
a specific medication is unpredictable. For this reason, a range of NSAIDs may
be tried in each case with the most effective preparation being continued.
Patients should be closely monitored for adverse reactions. The US Food and
Drug Administration advises that many NSAIDs may cause an increased risk of
serious cardiovascular thrombotic events, myocardial infraction, and stroke,
which can be fatal. Naproxen sodium does not appear to be associated with
increased risk of vascular events. Administration of proton pump inhibitors,
histamine 2 blockers, or prostaglandin analog misoprostol along with these
NSAIDs may reduce the risk of duodenal and gastric ulceration but do not impact
possible cardiovascular complications. Due to the cross-reactivity between
aspirin and NSAIDs, NSAIDs should not be used in aspirin-sensitive patients,
and should be used with caution in all asthma patients. NSAIDs maybe associated
with abnormal renal function, including renal failure, as well as, abnormal
liver function. Certain NSAIDs may have interactions with various other
medications. Individuals may have adverse events not listed above. Intervals
for metabolic screening are dependent upon the patient's age, general health
status and should be within parameters listed for each specific medication.
Complete Blood Count (CBC) and liver and renal function should be monitored at
least every six months in patients on chronic NSAIDs and initially when
indicated.
6.5.4.1 Non-selective
Nonsteroidal Anti-Inflammatory Drugs – Includes NSAIDs and acetylsalicylic acid
(aspirin). Serious GI toxicity, such as bleeding, perforation, and ulceration
can occur at any time, with or without warning symptoms in patients treated
with traditional NSAIDs. Physicians should inform patients about the signs
and/or symptoms of serious gastrointestinal toxicity and what steps to take if
they occur. Anaphylactoid reactions may occur in patients taking NSAIDs. NSAIDs
may interfere with
platelet
function. Fluid retention and edema have been observed in some patients taking
NSAIDs.
6.5.4.2 Selective
Cyclo-oxygenase-2 (COX-2) Inhibitors – COX-2 inhibitors are more recent NSAIDs
and differ in adverse side effect profiles from the traditional NSAIDs. The
major advantages of selective COX-2 inhibitors over traditional NSAIDs are that
they have less gastrointestinal toxicity and no platelet effects. COX-2 inhibitors
can worsen renal function in patients with renal insufficiency, thus renal
function may need monitoring.
COX-2
inhibitors should not be first-line for low risk patients who will be using an
NSAID
short-term
but are indicated in select patients for whom traditional NSAIDs are not
tolerated. Serious upper GI adverse events can occur even in asymptomatic
patients. Patients at high risk for GI bleed include those who use alcohol,
smoke, are older than 65, take corticosteroids or anti-coagulants, or have a longer
duration of therapy. Celecoxib is contraindicated in sulfanilamide allergic
patients.
6.5.5 Psychotropic/Anti-anxiety/Hypnotic
Agents: may be useful for treatment of mild and chronic pain, dysesthesias,
sleep disorders, and depression. Antidepressant medications, such as tricyclics
and Selective Serotonin Reuptake Inhibitors (SSRIs), are useful for affective
disorders and chronic pain management. Tricyclic antidepressant agents, in low
dose, are useful for chronic pain but have more frequent side effects.
Anti-anxiety
medications are best used for short-term treatment (i.e., less than 6 months).
Accompanying sleep disorders are best treated with sedating antidepressants
prior to bedtime. Frequently, combinations of the above agents are useful. As a
general rule, physicians should assess the patient for a prior history of
substance abuse or depression prior to prescribing any of these agents.
6.5.6 Tramadol:
is useful in relief of upper extremity pain and has been shown to provide
pain relief equivalent to that of commonly prescribed narcotics. Although
Tramadol may cause impaired alertness, it is generally well tolerated, does not
cause gastrointestinal ulceration, or exacerbate hypertension or congestive
heart failure. Tramadol should be used cautiously in patients who have a
history of seizures or who are taking medication that may lower the seizure
threshold, such as monoamine oxidase (MAO) inhibiters, SSRIs, and tricyclic
antidepressants. This medication has physically addictive properties and withdrawal
may follow abrupt discontinuation. It is not recommended for those with prior
opioid addiction.
6.5.7 Topical
Drug Delivery: may be an alternative treatment for localized
musculoskeletal disorders and is an acceptable form of treatment in selected patients
although there is no scientific evidence to support its use. It is necessary
that all topical agents be used with strict instructions for application as
well as maximum number of applications per day to obtain the desired benefit
and avoid potential toxicity. As with all medications, patient selection must
be rigorous to choose those patients with the highest probability of
compliance. Refer to “Iontophoresis” in the Passive Therapy section for
information regarding topical iontophoretic agents.
6.6 OCCUPATIONAL
REHABILITATION PROGRAMS
6.6.1 Non-Interdisciplinary:
These programs are work-related, outcome-focused, individualized treatment
programs. Objectives of the program include, but are not limited to, improvement
of cardiopulmonary and neuromusculoskeletal functions (strength, endurance,
movement, flexibility, stability, and motor control functions), patient
education, and symptom relief. The goal is for patients to gain full or optimal
function and return-to-work. The service may include the time-limited use of
passive modalities with progression to achieve treatment and/or simulated/real
work.
6.6.1.1 Work
Conditioning/Simulation
This program may begin once a patient is out of the acute phase of
injury and will be able to tolerate this program. These programs are usually
initiated after the acute phase has been completed and
offered at any time throughout the recovery phase. Work conditioning
should be initiated when imminent return of a patient to modified or full duty
is not an option, but the prognosis for returning the patient to work at
completion of the program is at least fair to good.
The need for work place simulation should be based upon the results of a
Functional Capacity Evaluation and/or Jobsite Analysis.
·
Length of visit:
1 to 4 hours per day.
·
Frequency: 2 to 5
visits per week
·
Maximum duration:
8 weeks. Participation in a program beyond six weeks must be documented with
respect to need and the ability to facilitate positive symptomatic or
functional gains.
6.6.1.2 Work
Hardening Work Hardening is an interdisciplinary program addressing a patient’s
employability and return to work. It includes a progressive increase in the
number of hours per day that a patient completes work simulation tasks until
the patient can tolerate a full workday. This is
accomplished by addressing the medical, psychological, behavioral,
physical, functional,
and vocational components of employability and return-to-work.
This can include a highly structured program involving a team approach or can
involve any
of the components thereof. The interdisciplinary team should, at a
minimum, be comprised of a qualified medical director who is board certified
with documented training in occupational rehabilitation; team physicians having
experience in occupational rehabilitation; occupational therapist; physical
therapist; case manager; and psychologist. As appropriate, the team may also
include: chiropractor, RN, vocational specialist or Certified Biofeedback
Therapist.
·
Length of visit:
Up to 8 hours/day
·
Frequency: 2 to 5
visits per week
·
Maximum duration:
8 weeks. Participation in a program beyond six weeks must be documented with
respect to need and the ability to facilitate positive symptomatic or functional
gains.
6.7 PATIENT EDUCATION
No treatment plan is complete without addressing issues of individual patient
and/or group education as a means of prolonging the beneficial effects of
treatment, as well as facilitating self-management of symptoms and injury
prevention. The patient should take an active role in the establishment of
functional outcome goals, and should be educated on his or her specific injury,
assessment findings, and plan of treatment. Education and instruction in proper
body mechanics and posture, positions to avoid task/tool adaptation, self-care
for exacerbation of symptoms, and home exercise/task adaptation should also be
addressed.
6.8 RETURN-TO-WORK is
therapeutic, assuming the work is not likely to aggravate the basic problem or
increase long-term pain. The practitioner must provide specific physical
limitations per the Physician’s Form. The following physical limitations should
be considered and modified as recommended: lifting, pushing, pulling,
crouching, walking, using stairs, bending at the waist, awkward and/or
sustained postures, tolerance for sitting or standing, hot and cold
environments, data entry and other repetitive motion tasks, sustained grip,
tool usage and vibration factors. Even if there is residual chronic pain,
return-to-work is not necessarily contraindicated.
The practitioner should understand all of
the physical demands of the patient’s job position before returning the patient
to full duty and should receive clarification of the patient’s job duties.
Clarification must be provided by the employer or, if necessary, including, but
not limited to, an occupational health nurse, occupational therapist,
vocational rehabilitation specialist, or an industrial hygienist.
6.9 SLEEP
DISTURBANCES are a common secondary symptom of CTD. Although primary
insomnia may accompany pain as an independent co-morbid condition, it more
commonly occurs, secondary to the pain condition itself. Exacerbations of pain
often are accompanied by exacerbations of insomnia; the reverse can also occur.
Sleep laboratory studies have shown disturbances of sleep architecture in pain
patients. Loss of deep slow-wave sleep and increase in light sleep occur and
sleep efficiency, the proportion of time in bed spent asleep, is decreased.
These changes are associated with patient reports of non-restorative sleep.
Many affected patients develop behavioral
habits that exacerbate and maintain sleep disturbances. Excessive time in bed,
irregular sleep routine, napping, low activity, and worrying in bed are all
maladaptive responses that can arise in the absence of any psychopathology.
There is some evidence that behavioral modification, such as patient education
and group or individual counseling, can be effective in reversing the effects
of insomnia. Behavioral modifications are easily implemented and can include:
6.9.1 Maintaining
a regular sleep schedule, retiring and rising at approximately the same time on
weekdays and weekends.
6.9.2 Avoiding
daytime napping.
6.9.3 Avoiding
caffeinated beverages after lunchtime
6.9.4 Making
the bedroom quiet and comfortable, eliminating disruptive lights, sounds,
television sets, and keeping a bedroom temperature of about 65°F.
6.9.5 Avoiding
alcohol or nicotine within two hours of bedtime.
6.9.6 Avoiding
large meals within two hours of bedtime.
6.9.7 Exercising
vigorously during the day, but not within two hours of bedtime, since this may
raise core temperature and activate the nervous system.
6.9.8 Associating
the bed with sleep and sexual activity only, using other parts of the home for
television, reading and talking on the telephone.
6.9.9 Leaving
the bedroom when unable to sleep for more than 20 minutes, retuning to the
bedroom
when
ready to sleep again.
These modifications should be undertaken before sleeping medication is
prescribed for long term
use.
6.10 THERAPY–PASSIVE
includes those treatment modalities that do not require energy expenditure
on the part of the patient. They are principally effective during the early
phases of treatment and are directed at controlling symptoms such as pain,
inflammation and swelling and to improve the rate of healing soft tissue
injuries. They should be used in adjunct with active therapies to help control
swelling, pain and inflammation during the rehabilitation process. They may be
used intermittently as a therapist deems appropriate or regularly if there are
specific goals with objectively measured functional improvements during
treatment.
6.10.1 Electrical
Stimulation (Unattended and Attended): once applied, requires minimal
on-site supervision by the physician or non-physician provider. Indications
include pain, inflammation, muscle spasm, atrophy, and decreased circulation.
6.10.2 Extracorporeal
shock wave treatment: Consists of the application of pulses of high
pressure sound to soft tissues, similar to lithotriptors. It has been
investigated for its effectiveness in the treatment of lateral epicondylitis.
It has not been shown to have an advantage over other conservative treatments
and remains investigational. It is not recommended.
6.10.3 Iontophoresis: is the
transfer of medication, including, but not limited to, steroidal antiinflammatories
and anesthetics, through the use of electrical stimulation. Indications include
pain (Lidocaine), inflammation (hydrocortisone, salicylate), edema (mecholyl,
hyaluronidase, salicylate), ischemia (magnesium, mecholyl, iodine), muscle
spasm (magnesium, calcium), calcific deposits (acetate), scars and keloids
(chlorine, iodine, acetate).
6.10.4 Laser irradiation:
Consists of the external application of an array of visible and infrared
wavelengths to soft tissues. Time and frequency dependent on severity and
chronicity of problem.
6.10.5 Manual Therapy Techniques:
are passive interventions in which the providers use his or her hands to
administer skilled movements designed to modulate pain; increase joint range of
motion; reduce/eliminate soft tissue swelling, inflammation, or restriction;
induce relaxation; and improve contractile and non-contractile tissue
extensibility. These techniques are applied only after a thorough examination
is performed to identify those for whom manual therapy would be contraindicated
or for whom manual therapy must be applied with caution.
6.10.5.1 Manipulation:
is generally accepted, well-established and widely used therapeutic
intervention for low back pain. Manipulative Treatment (not therapy) is defined
as the therapeutic application of manually guided forces by an operator to
improve physiologic function and/or support homeostasis that has been altered
by the injury or occupational disease, and has associated clinical
significance.
High velocity, low amplitude (HVLA) technique, chiropractic
manipulation, osteopathic manipulation, muscle energy techniques, counter
strain, and non-force techniques are all types of manipulative treatment. This
may be applied by osteopathic physicians (D.O.), chiropractors (D.C.), properly
trained physical therapists (P.T.), or properly trained medical physicians.
Under these different types of manipulation exist many subsets of different
techniques that can be described as
6.10.5.1.1 direct-
a forceful engagement of a restrictive/pathologic barrier,
6.10.5.1.2 indirect-
a gentle/non-forceful disengagement of a restrictive/pathologic barrier,
6.10.5.1.3 the
patient actively assists in the treatment and
6.10.6.1.4 the
patient relaxing, allowing the practitioner to move the body tissues. When the
proper diagnosis is made and coupled with the appropriate technique,
manipulation has no contraindications and can be applied to all tissues of the
body.
Pre-treatment assessment should be performed as part of each
manipulative treatment visit to ensure that the correct diagnosis and correct
treatment is employed. High velocity, low amplitude (HVLA) manipulation is
performed by taking a joint to its
end range of motion and moving the articulation into the zone of
accessory joint movement, well within the limits of anatomical integrity.
Indications for manipulation include joint pain, decreased joint motion, and
joint adhesions. Contraindications to HVLA manipulation include joint
instability, fractures, severe osteoporosis, infection, metastatic cancer,
active inflammatory arthritides, aortic aneurysm, and signs of progressive
neurologic deficits.
·
Time to produce
effect for all types of manipulative treatment: 1 to 6 treatments.
·
Frequency: Up to
3 times per week for the first 4 weeks as indicated by the severity of involvement
and the desired effect, then up to 2 treatments per week for the next 4 weeks.
For further treatments, twice per week or less to maintain function.
·
Maximum duration:
30 visits. Extended durations of care beyond what is considered “maximum” may
be necessary in cases of re-injury, interrupted continuity of care,
exacerbation of symptoms, and in those patients with comorbidities. Refer to
the Chronic Pain Guidelines for care beyond 6 months.
The combination of 97140 plus either CMT or
OMT code is equal to one visit when performed on the same day. Any combination
of manual therapeutic intervention exceeding 30 visits (not units) need to go
to UR.
6.10.5.2 Mobilization
(Joint) /Manipulation Mobilization is passive movement involving
oscillatory motions to the involved joints. The passive mobility is performed
in a graded manner (I, II, III, IV, or V), which depicts the speed of the
maneuver. It may include skilled manual joint tissue stretching. Indications
include the need to improve joint play,
improve intracapsular arthrokinematics, or reduce pain associated with tissue
impingement.
·
Time to produce
effect: 4 to 6 treatments
·
Frequency: 2 to 3
times per week
·
Maximum duration:
30 visits (CPT codes 97124 and 97140 can not exceed 30 visits in combination).
6.10.5.3 Mobilization
(Soft Tissue) Mobilization of soft tissue is the skilled application of
manual techniques designed to normalize movement patterns through the reduction
of soft tissue pain and restrictions.
Indications include muscle spasm around a
joint, trigger points, adhesions, and neural
compression.
Nerve Gliding: consist of a series of flexion and
extension movements of the hand, wrist,
elbow, shoulder, and neck that produce
tension and longitudinal movement along the length of the median and other
nerves of the upper extremity. These exercises are based on the principle that
the tissues of the peripheral nervous system are designed for movement, and
that tension and glide (excursion) of nerves may have an effect on
neurophysiology through alterations in vascular and axoplasmic flow.
Biomechanical principles have been more thoroughly studied than clinical
outcomes. Nerve gliding performed on a patient by the clinician should be
reinforced by patient performance of similar techniques as part of a home
exercise program at least twice per day.
·
Time to produce
effect: 4 to 6 treatments
·
Frequency: 2 to 3
times per week
·
Maximum duration:
30 visits (CPT codes 97124 and 97140 can not exceed 30 visits in combination).
6.10.6 Massage:
Manual or Mechanical - Massage is manipulation of soft tissue with broad
ranging relaxation and circulatory benefits. This may include stimulation of
acupuncture points and acupuncture channels (acupressure), application of
suction cups and techniques that include pressing, lifting, rubbing, pinching
of soft tissues by or with the practitioners’ hands. Indications include edema,
muscle spasm, adhesions, the need to improve peripheral circulation and range
of motion, or to increase muscle relaxation and flexibility prior to exercise.
·
Time to produce
effect: Immediate.
·
Frequency: 1 to 3
times per week
·
Maximum duration:
12 visits (CPT codes 97124 and 97140 can not exceed 30 visits in combination).
6.10.7 Orthotics/Immobilization
with Splinting: is a generally accepted, well-established and widely used
therapeutic procedure. Splints may be effective when worn at night or during
portions of the day, depending on activities. Splints should be loose and soft
enough to maintain comfort while supporting the involved joint in a relatively
neutral position. Splint comfort is critical and may affect compliance.
Although off-the-shelf splints are usually sufficient, custom thermoplastic
splints may provide better fit for certain patients.
Splints may be effective when worn at night
or during portions of the day, depending on activities; however, splint use is
rarely mandatory. Providers should be aware that over-usage is
counterproductive, and counsel patients to minimize daytime splint use in order
avoid detrimental effects, such as, stiffness and dependency over time.
·
Time to produce
effect: 1-4 weeks
·
Frequency:
Daytime intermittent or night use, depending on symptoms and activities.
·
Maximum duration:
2 to 4 months. If symptoms persist, consideration should be given to further
diagnostic studies or to other treatment options.
6.10.8 Superficial
Heat and Cold Therapy: are thermal agents applied in various manners that
lowers or raises the body tissue temperature for the reduction of pain,
inflammation, and/or effusion resulting from injury or induced by exercise.
Includes application of heat just above the surface of the skin at acupuncture
points. Indications include acute pain, edema and hemorrhage, need to increase
pain threshold, reduce muscle spasm and promote stretching/flexibility. Cold
and heat packs can be used at home as an extension of therapy in the clinic
setting.
·
Time to produce
effect: Immediate
·
Frequency: 2 to 5
times per week (clinic). Home treatment as needed.
·
Maximum duration:
12 visits, with a maximum visit 1 per day. If symptoms persist, consideration should be given to further
diagnostic studies or other treatment options.
6.10.9 Ultrasound:
uses sonic generators to deliver acoustic energy for therapeutic thermal
and/or nonthermal soft tissue effects. Indications include scar tissue,
adhesions, collagen fiber and muscle spasm, and to improve muscle tissue
extensibility and soft tissue healing. Ultrasound with electrical stimulation
is concurrent delivery of electrical energy that involves dispersive electrode
placement. Indications include muscle spasm, scar tissue, pain modulation and
muscle facilitation. Phonophoresis is the transfer of medication to the target
tissue to control inflammation and pain through the use of sonic generators.
These topical medications include, but are not limited to, steroidal
anti-inflammatory and anesthetics.
·
Time to produce
effect: 4 to 8 treatments
·
Frequency: 2-3
times per week
·
Maximum duration:
18 visits
6.11 THERAPY–ACTIVE
therapies are based on the philosophy that therapeutic exercise and/or activity
are beneficial for restoring flexibility, strength, endurance, function, range
of motion, and alleviating discomfort. Active therapy requires an internal
effort by the individual to complete a specific exercise or task, and thus
assists in developing skills promoting independence to allow self-care after
discharge. This form of therapy requires supervision from a therapist or medical
provider such as verbal, visual, and/or tactile instructions. At times a
provider may help stabilize the patient or guide the movement pattern but the
energy required to complete the task is predominately executed by the patient.
Patients should be instructed to continue
active therapies at home as an extension of the treatment process in order to
maintain improvement levels. Home exercise can include exercise with or without
mechanical assistance or resistance and functional activities with assistive
devices.
Interventions are selected based on the
complexity of the presenting dysfunction with ongoing examination, evaluation
and modification of the plan of care as improvement or lack thereof occurs.
Change and/or discontinuation of an intervention should occur if there is
attainment of expected goals/ outcome, lack of progress, lack of tolerance
and/or lack of motivation. Passive interventions/modalities may only be used as
adjuncts to the active program.
6.11.1 Activities
of Daily Living: Supervised instruction, active-assisted training, and/or
adaptation of activities or equipment to improve a person’s capacity in normal
daily living activities such as self-care, work re-integration training,
homemaking, and driving.
·
Time to produce
effect: 4 to 5 treatments
·
Maximum of 10
sessions
6.11.2 Aquatic
Therapy: is a well-accepted treatment which consists of the therapeutic use
of aquatic immersion for therapeutic exercise to promote strengthening, core
stabilization, endurance, range of motion, flexibility, body mechanics, and
pain management. Aquatic therapy includes the implementation of active
therapeutic procedures in a swimming or therapeutic pool. The water provides a
buoyancy force that lessens the amount of force gravity applies to the body.
The decreased gravity effect allows the patient to have a mechanical advantage
and more likely have a successful trial of therapeutic exercise. The therapy
may be indicated for individuals who:
·
cannot tolerate
active land-based or full-weight bearing therapeutic procedures
·
require increased
support in the presence of proprioceptive deficit;
·
are at risk of
compression fracture due to decreased bone density;
·
have symptoms
that are exacerbated in a dry environment;
·
would have a
higher probability of meeting active therapeutic goals than in a land-
§
based
environment. The pool should be large enough to allow full extremity range of
motion and fully erect posture. Aquatic vests, belts and other devices can be
used to provide stability, balance, buoyancy, and resistance.
·
Time to produce
effect: 4 to 5 treatments
·
Frequency: 3 to 5
times per week
·
Maximum duration:
24 visits
A
self-directed program is recommended after the supervised aquatics program has
been established, or, alternatively a transition to a land-based environment
exercise program.
6.11.3 Functional Activities:
are the use of therapeutic activity to enhance mobility, body mechanics,
employability, coordination, and sensory motor integration.
·
Time to produce
effect: 4 to 5 treatments
·
Frequency: 3 to 5
times per week
• Maximum duration: 24 visits Total number of visit
97110 and 97530 should not exceed 36 visits without pre-authorization
6.11.4 Neuromuscular
Re-education: is the skilled application of exercise with manual,
mechanical, or electrical facilitation to enhance strength, movement patterns,
neuromuscular response, proprioception, kinesthetic sense, coordination
education of movement, balance, and posture. Indications include the need to
promote neuromuscular responses through carefully timed proprioceptive stimuli,
to elicit and improve motor activity in patterns similar to normal
neurologically developed sequences, and improve neuromotor response with
independent control.
·
Time to produce
effect: 2 to 6 treatments
·
Frequency: 3-5
times per week
·
Maximum duration:
24 visits
6.11.5 Proper Work Techniques:
Please refer to the “Job Site Evaluation” and “Job Site Alteration” sections of
these guidelines.
6.11.6 Therapeutic Exercise:
with or without mechanical assistance or resistance may include isoinertial,
isotonic, isometric and isokinetic types of exercises. Indications include the
need for cardiovascular fitness, reduced edema, improved muscle strength,
improved connective tissue strength and integrity, increased bone density,
promotion of circulation to enhance soft tissue healing, improvement of muscle
recruitment, increased range of motion, and are used to promote normal movement
patterns. Can also include complementary/alternative exercise movement therapy.
·
Time to produce
effect: 2 to 6 treatments
·
Frequency: 3 to 5
times per week
• Maximum duration: 36 visits Total number of visit
97110 and 97530 should not exceed 36 visits without pre-authorization
6.12 RESTRICTION OF
ACTIVITIES Continuation of normal daily activities is the recommendation
for Cumulative Trauma Disorders with or without neurologic symptoms. Complete
work cessation should be avoided, if possible, since it often further
aggravates the pain presentation. Modified return-to-work is almost always more
efficacious and rarely contraindicated in the vast majority of injured workers
with CTD.
6.13 VOCATIONAL
REHABILITATION is a generally accepted intervention. Initiation of
vocational rehabilitation requires adequate evaluation of patients for
quantification of highest functional level, motivation, and achievement of
maximum medical improvement. Vocational rehabilitation may be as simple as
returning to the original job or as complicated as being retrained for a new
occupation.
THE FOLLOWING SURGICAL GUIDELINES ARE NOT
INTENDED TO REPLACE THE SURGEON’S JUDGMENT.
Operative treatment may be indicated when
the individual component diagnoses that make up CTD prove unresponsive to the
full complement of non-operative options, including job site analysis and
modification. Physical exam findings should be well localized and consistent
with the diagnosis. Severe neurologic findings are an exception to these
indications, and may suggest earlier surgical intervention. Surgical results
must anticipate objective functional gains and improved activities of daily
living.
Surgery in CTD usually falls into two broad
categories: peripheral nerve decompression and muscle or tendon sheath release
or debridement. The treating surgeon must determine the appropriate procedure
and timing for the individual case. The most common surgical procedures that
are performed in CTD patients are listed below; other procedures may be
indicated in certain cases.
Since CTD often involves several areas in an
upper extremity, surgical treatment of one problem should be performed in
conjunction with conservative treatment of other problems in the upper
extremity.
7.1 PERIPHERAL
NERVE DECOMPRESSION Surgery may be considered when findings on history and
physical exam correlate specifically with the diagnosis being considered.
Subjective complaints should be localized and appropriate to the diagnosis,
neurologic complaints should be consistent with the nerve distribution in
question, and physical exam findings should correlate with the history. Surgery
may be considered as an initial therapy in situations where there is clinical
and/or electrodiagnostic evidence of severe or progressive neuropathy.
Objective evidence should be present in all cases in which surgery is
contemplated. Objective evidence may include: electrodiagnostic (EDX) studies,
diagnostic peripheral nerve block which eradicates the majority of the
patient’s symptoms, or a motor deficit commensurate with the suspected
neurologic lesion. Refer to Physical Examination Findings (section D.2,
physical examination) for objective diagnostic findings. Job modification
should be considered prior to surgery. Refer to the “Job Site Alteration”
section for additional information on job modification.
When no objective evidence is present and
the patient continues to have signs and symptoms consistent with the diagnosis
after six months of conservative treatment including a psychological
evaluation, a second opinion should be obtained before operative treatment is
considered.
Specific procedures and their indications
are outlined below:
7.1.1 Median
Nerve Decompression at the Wrist (carpal tunnel release): Please refer to
the Division’s, Carpal Tunnel Syndrome Medical Treatment Guidelines.
7.1.2 Median
Nerve Decompression in the Forearm (pronator teres or flexor digitorum
superficialis release): Please refer to Physical Examination Findings Table
(section D.2, physical examination) Electrodiagnostic (EDX) studies may show
delayed median nerve conduction in the forearm. If nerve conduction velocity is
normal with suggestive clinical findings, the study may be repeated after a 3-6
month period of continued conservative treatment. If the study is still normal,
the decision on treatment is made on the consistency of clinical findings and
the factors noted above.
7.1.3 Ulnar
Nerve Decompression at the Wrist (ulnar tunnel release or Guyon’s canal
release) Please refer to Physical Examination Findings Reference Table
(section D.2, physical examination) Electrodiagnostic testing may confirm the
diagnosis and differentiate from ulnar entrapment neuropathy at the elbow.
7.1.4 Ulnar
Nerve Decompression/Transposition at the Elbow: Please refer to Physical
Examination Findings Reference Table (section D.2, physical examination)
Electrodiagnostic studies (EDX) may indicate an ulnar neuropathy at the elbow.
In general, patients with minimal symptoms or without objective findings of
weakness tend to respond better to conservative treatment than patients with
measurable pinch or grip strength weakness. If objective findings persist
despite conservative treatment, surgical options include: simple decompression,
medial epicondylectomy with decompression, anterior subcutaneous transfer, and
submuscular or intramuscular transfer.
7.1.5 Sensory
Nerve Decompression at the Wrist: Please refer to Physical Examination
Findings Reference Table (section D.2, physical examination) of these
guidelines. Electrodiagnostic (EDX) studies can be useful in establishing a
diagnosis but negative studies do not exclude the diagnosis
7.1.6 Radial
Nerve Decompression at the Elbow: Please refer to Physical Examination
Findings Reference Table (section D.2, physical examination) Electrodiagnostic
(EDX) studies are helpful when positive, but negative studies do not exclude
the diagnosis.
7.1.7 Thoracic
Outlet Syndrome: Please refer to the Division’s Thoracic Outlet Syndrome
Medical Treatment Guidelines.
7.2 TENDON
DECOMPRESSION OR DEBRIDEMENT Surgery may be considered when several months
of appropriate treatment have failed, and findings on history and physical exam
correlate specifically with the diagnosis being considered. Subjective
complaints should be localized and appropriate to the diagnosis, and physical
exam findings should correlate with the history. Refer to the Physical
Examination Findings Table (section D.2, physical examination). Job
modification should be considered prior to surgery. Refer to Job Site
Alteration (Section F.4) for additional information on job modification.
Specific procedures and their indications
are outlined below:
7.2.1 Subacromial
Decompression: Please refer to the Division’s Shoulder Injury Medical
Treatment Guidelines.
7.2.2 Medial
or Lateral Epicondyle Release/Debridement: Please refer to Physical
Examination Findings Reference Table (section D.2, physical examination). It is
generally accepted that 80% of cases improve with conservative therapy.
Intermittent discomfort may recur over six months to one year after initial
conservative treatment. Surgery should only be performed to achieve functional
gains on those with significant ongoing impaired activities of daily living.
X-rays may be normal or demonstrate spur formation over the involved
epicondyle.
7.2.3 First
Extensor Compartment Release (de Quervain’s Tenosynovitis): Please refer to
Physical Examination Findings Reference Table (section D.2, physical
examination). Surgery should be performed to achieve functional gains on those
with significant ongoing impaired activities of daily living.
7.2.4 Trigger
Finger/Thumb Release: Please refer to Physical Examination Findings Reference
Table (section D.2, physical examination). Surgery should be performed to
achieve functional gains on those with significant ongoing impaired activities
of daily living.
7.3 CONSIDERATIONS
FOR POST-OPERATIVE THERAPY
7.3.1 Immobilization:
Controlled mobilization, and/or formal physical/occupational therapy should
begin as soon as possible following surgery at the discretion of the treating
surgeon. Final decisions regarding the
need for splinting post-operatively should be left to the discretion of the
treating physician based upon his/her understanding of the surgical technique
used and the specific conditions of the patient.
7.3.2 Home
Program: It is generally accepted that all patients should receive a home
therapy protocol involving stretching, ROM, scar care, and resistive exercises.
Once they have been cleared for increased activity by the surgeon, patients
should be encouraged to use the hand as much as possible for daily activities,
allowing pain to guide their level of activity.
7.3.3 Supervised
Therapy Program: may be helpful in patients who do not show functional
improvements post-operatively or in patients with heavy or repetitive job
activities. The therapy program may include some of the generally accepted
elements of soft tissue healing and return to function:
7.3.3.1 Soft
tissue healing/remodeling: May be used after the incision has healed. It may
include any of the following: evaluation, whirlpool, electrical stimulation,
soft tissue mobilization, scar compression pad, heat/cold application,
splinting or edema control may be used as indicated. Following wound
healing, ultrasound and iontophoresis with
sodium chloride (NaCl) may be considered for soft tissue remodeling. Diathermy
is not an acceptable adjunct.
7.3.3.2 Return
to function: Range of motion and stretching exercises, strengthening, activity
of daily living
adaptations,
joint protection instruction, posture/body mechanics education. Job site
modifications may be indicated.
·
Time to produce
effect: 2-4 weeks
·
Frequency: 2-5
times/week
·
Maximum duration:
36 visits
PART D LOW BACK TREATMENT GUIDELINES
Pursuant to 19 Del.C. §2322C, health
care practice guidelines have been adopted and recommended by the Health Care
Advisory Panel to guide utilization of health care treatments in workers'
compensation including, but not limited to, care provided for the treatment of
employees by or under the supervision of a licensed health care provider,
prescription drug utilization, inpatient hospitalization and length of stay,
diagnostic testing, physical therapy, chiropractic care and palliative care.
The health care practice guidelines apply to all treatments provided after the
effective date of the regulation adopted by the Department of Labor, May 23,
2008, and regardless of the date of injury. The guidelines are, to the extent
permitted by the most current medical science or applicable science, based on
well-documented scientific research concerning efficacious treatment for
injuries and occupational disease. To the extent that well-documented
scientific research regarding the above is not available at the time of
adoption of the guidelines, or is not available at the time of any revision to
the guidelines, the guidelines have been and will be based upon the best
available information concerning national consensus regarding best health care
practices in the relevant health care community.
The
guidelines, to the extent practical and consistent with the Act, address
treatment of those physical conditions which occur with the greatest frequency,
or which require the most expensive treatments, for work-related injuries based
upon currently available Delaware
data.
Services
rendered by any health care provider certified pursuant to 19 Del.C.
§2322D(a) to provide treatment or services for injured employees shall be
presumed, in the absence of contrary evidence, to be reasonable and necessary
if such treatment and/or services conform to the most current version of the
Delaware health care practice guidelines.
Services
rendered outside the Guidelines and/or variation in treatment recommendations
from the Guidelines may represent acceptable medical care, be considered
reasonable and necessary treatment and, therefore, determined to be
compensable, absent evidence to the contrary, and may be payable in accordance
with the Fee Schedule and Statute, accordingly.
Services
provided by any health care provider that is not certified pursuant to 19 Del.C.
§2322D(a) shall not be presumed reasonable and necessary unless such services
are pre-authorized by the employer or insurance carrier, subject to the
exception set forth in 19 Del.C. §2322D(b).
Treatment
of conditions unrelated to the injuries sustained in an industrial accident may
be denied as unauthorized if the treatment is directed toward the
non-industrial condition, unless the treatment of the unrelated injury is
rendered necessary as a result of the industrial accident.
The
Health Care Advisory Panel and Department of Labor recognized that acceptable
medical practice may include deviations from these Guidelines, as individual
cases dictate. Therefore, these Guidelines are not relevant as evidence of a
provider's legal standard of professional care.
In accordance with the requirements of the
Act, the development of the health care guidelines has been directed by a
predominantly medical or other health professional panel, with recommendations
then made to the Health Care Advisory Panel.
2.1 TREATMENT
PARAMETER With respect to Therapy (Active or Passive), time frames/visits
for specific interventions commence once treatments have been initiated, not on
the date of injury. Obviously, duration will be impacted by patient compliance,
as well as comorbitities and availability of services. Clinical judgment may substantiate
the need to accelerate or decelerate modify the time frames total number of
visits discussed in this document. The majority of injured workers with low
back pain often will achieve resolution of their condition within 8 to 24
visits (Guide to Physical Therapy Practice – Second Edition). It is anticipated that most injured workers
will not require the maximum number of visits described in these
guidelines. They are designed to be a
ceiling and care extending beyond the maximum allowed visits may warrant
utilization review.
2.2 ACTIVE
INTERVENTIONS emphasizing patient responsibility, such as therapeutic
exercise and/or functional treatment, are generally emphasized over passive
modalities, especially as treatment progresses. Generally, passive
interventions are viewed as a means to facilitate progress in an active
rehabilitation program with concomitant attainment of objective functional
gains. All rehabilitation programs must incorporate “Active Interventions” no later
than twelve visits three weeks after the onset of treatment. Reimbursement for
passive modalities only after the first twelve visits three weeks of treatment
without clear evidence of Active Interventions will require supportive
documentation.
2.3 ACTIVE
THERAPEUTIC EXERCISE PROGRAM goals should incorporate patient strength,
endurance, flexibility, coordination, and education. This includes functional
application in vocational or community settings.
2.4 POSITIVE
PATIENT RESPONSE results are defined primarily as functional gains that can
be objectively measured. Objective functional gains include, but are not
limited to, positional tolerances, range of motion (ROM), strength, endurance,
activities of daily living, cognition, behavior, and efficiency/velocity
measures that can be quantified. Subjective reports of pain and function should
be considered and given relative weight when the pain has anatomic and
physiologic correlation.
2.5 RE-EVALUATE
TREATMENT EVERY 3 TO 4 WEEKS With respect to Therapy (Active or Passive),
if a given treatment or modality is not producing positive results within 3 to
4 weeks, the treatment may be either modified or discontinued. Reconsideration
of diagnosis should also occur in the event of poor response to a seemingly rational
intervention.
2.6 SURGICAL
INTERVENTIONS should be contemplated within the context of expected
functional outcome and not purely for the purpose of pain relief. The concept
of “cure” with respect to surgical treatment by itself is generally a misnomer.
All operative interventions must be based upon positive correlation of clinical
findings, clinical course, and diagnostic tests. A comprehensive assimilation
of these factors must lead to a specific diagnosis with positive identification
of pathologic conditions.
2.7 SIX-MONTH
TIME FRAME The prognosis drops precipitously for returning an injured
worker to work once he/she has been temporarily totally disabled for more than
six months. The emphasis within these guidelines is to move patients along a
continuum of care and return to work within a six-month time frame, whenever
possible. It is important to note that time frames may not be pertinent to
injuries that do not involve work-time loss or are not occupationally related.
2.8 RETURN-TO-WORK
Is therapeutic, assuming the work is not likely to aggravate the basic problem
or increase long-term pain. The following physical limitations should be
considered and modified as recommended: lifting, pushing, pulling, crouching,
walking, using stairs, bending at the waist, awkward and/or sustained postures,
tolerance for sitting or standing, hot and cold environments, data entry and
other repetitive motion tasks, sustained grip, tool usage and vibration
factors. Even if there is residual chronic pain, return-to-work is not
necessarily contraindicated.
The practitioner should understand all of
the physical demands of the patient’s job position before returning the patient
to full duty and should receive clarification of the patient’s job duties.
2.9 GUIDELINE
RECOMMENDATIONS AND INCLUSION OF MEDICAL EVIDENCE. Recommendations are
based on available evidence and/or consensus recommendations of the standard of
care within Delaware.
Those procedures considered inappropriate, unreasonable, or unnecessary are designated
in the guideline as being “not recommended.”
2.10 DELAYED
RECOVERY. The Department recognizes that not of all industrially injured
patients will recover within the time lines outlined in this document
despite optimal care. Such individuals may require treatments beyond the limits
discussed within this document, but such treatment will require clear
documentation by the authorized treating practitioner focusing on objective
functional gains afforded by further treatment and impact upon prognosis.
The remainder of this document should be
interpreted within the parameters of these guideline principles that may lead
to more optimal medical and functional outcomes for injured workers.
The Division recommends the following
diagnostic procedures be considered, at least initially, the responsibility of
the workers’ compensation carrier to ensure that an accurate diagnosis and
treatment plan can be established. Standard procedures, that should be utilized
when initially diagnosing a work-related low back pain complaint, are listed
below.
3.1 HISTORY-TAKING
AND PHYSICAL EXAMINATION (Hx & PE) are generally accepted,
well-established and widely used procedures that establish the foundation/basis
for and dictates subsequent stages of diagnostic and therapeutic procedures.
When findings of clinical evaluations and those of other diagnostic procedures
are not complementing each other, the objective clinical findings should have
preference. The medical records should reasonably document the following.
3.1.1 History
of Present Injury A detailed history, taken in temporal proximity to the
time of injury should primarily guide evaluation and treatment.
3.1.2 Past
History:
3.1.3 Physical
Examination: Should include accepted tests and exam techniques applicable
to the area being examined.
3.2 RADIOGRAPHIC
IMAGING of the lumbosacral spine is a generally accepted, well-established
and widely used diagnostic procedure when specific indications based on history
and/or physical examination are present. There is some evidence that early
radiographic imaging without clear indications is associated with prolonged
care, but no difference in functional outcomes. Therefore, it should not be
routinely performed without indications. The mechanism of injury and specific
indications for the radiograph should be listed on the request form to aid the
radiologist and x-ray technician. Suggested indications may include:
3.2.1 History
of significant trauma, especially blunt trauma or fall from a height;
3.2.2 Age
over 55 years;
3.2.3 Unexplained
or persistent low back pain for at least 6 weeks or pain that is worse with
rest;
3.2.4 Localized
pain, fever, constitutional symptoms, or history or exam suggestive of intravenous
drug abuse, prolonged steroid use, or osteomyelitis;
3.2.5 Suspected
lesion in the lumbosacral spine due to systemic illness such as a
rheumatic/rheumatoid disorder or endocrinopathy. Suspected lesions may require
special views;
3.2.6 Past
medical history suggestive of pre-existing spinal disease, osteoporosis, spinal
instrumentation, or cancer; and
3.2.7 Prior
to high-velocity/low amplitude manipulation or Grade IV to V mobilization.
3.3 LABORATORY
TESTING Laboratory tests are generally accepted, well-established and
widely used procedures. They are, however, rarely indicated at the time of
initial evaluation, unless there is suspicion of systemic illness, infection,
neoplasia, or underlying rheumatologic disorder, connective tissue disorder, or
based on history and/or physical examination. Laboratory tests can provide
useful diagnostic information. Tests include, but are not limited to:
3.3.1 Complete
blood count (CBC) with differential can detect infection, blood dyscrasias, and
medication side effects;
3.3.2 Erythrocyte
sedimentation rate (ESR), rheumatoid factor (RF), antinuclear antigen (ANA),
human leukocyte antigen (HLA), and C-reactive protein (CRP), can be used to
detect evidence of a rheumatologic, infectious, or connective tissue disorder;
3.3.3 Serum
calcium, phosphorous, uric acid, alkaline phosphatase, and acid phosphatase can
detect metabolic bone disease;
3.3.4 Urinalysis
for bacteria (usually with culture and sensitivity), calcium, phosphorus,
hydroxyproline, or hematuria; and
3.3.5 Liver and kidney function may be performed
for prolonged anti-inflammatory use or other medications requiring monitoring.
One diagnostic imaging procedure may provide
the same or distinctive information as does another procedure. Therefore, the
prudent choice of a single diagnostic procedure, a complement of procedures or
a sequence of procedures will optimize diagnostic accuracy; maximize cost
effectiveness (by avoiding redundancy), and minimize potential adverse effects
to patients.
All imaging procedures have a degree of
specificity and sensitivity for various diagnoses. No isolated imaging test can
assure a correct diagnosis. Clinical information obtained by history taking and
physical examination should form the basis for selecting an imaging procedure
and interpreting its results.
Magnetic resonance imaging (MRI),
myelography, or Computed Axial Tomography (CT) scanning following myelography
may provide useful information for many spinal disorders. When a diagnostic
procedure, in conjunction with clinical information, can provide sufficient
information to establish an accurate diagnosis, the second diagnostic procedure
will become a redundant procedure. At the same time, a subsequent diagnostic
procedure can be a complementary diagnostic procedure if the first or preceding
procedures, in conjunction with clinical information, cannot provide an
accurate diagnosis. Usually, preference of a procedure over others depends upon
availability, a patient’s tolerance, and/or the treating practitioner’s
familiarity with the procedure.
4.1 IMAGING
STUDIES are generally accepted, well-established and widely used diagnostic
procedures. In the absence of myelopathy, or progressive neurological changes,
or neurologic deficit, or history of cancer, imaging usually is not appropriate
until conservative therapy has been tried and failed. Four to six weeks of
treatment are usually an adequate period of time before an imaging procedure is
in order, but the clinician should use judgment in this regard. When indicated,
imaging studies can be utilized for further evaluation of the low back, based
upon the mechanism of injury, symptoms, and patient history. Prudent choice of
a single diagnostic procedure, a complementary combination of procedures, or a
proper sequential order of complementary procedures will help ensure maximum
diagnostic accuracy and minimize adverse effect to the patient. When the findings
of the diagnostic imaging and testing procedures are not consistent with the
clinical examination, the clinical findings should have preference.
The studies below are listed in frequency of
use, not importance:
4.1.1 Magnetic
Resonance Imaging (MRI): is rarely indicated in patients with non-traumatic
acute low back pain with no neuropathic signs or symptoms. It is generally the
first follow-up imaging study in individuals who respond poorly to proper
initial conservative care. MRI is useful in suspected nerve root compression,
myelopathy, masses, infections, metastatic disease, disc herniation, annular
tear, and cord contusion. MRI is contraindicated in patients with certain
implants.
In general, the high field, conventional,
MRI provides better resolution. A lower field scan may be indicated when a
patient cannot fit into a high field scanner or who is too claustrophobic
despite sedation. Inadequate resolution on the first scan may require a second
MRI using a different technique.
4.1.2 Computed
Axial Tomography (CT) provides excellent visualization of bone and is used
to further evaluate bony masses and suspected fractures and joints not clearly
identified on radiographic evaluation. It may sometimes be done as a complement
to MRI scanning to better delineate bony osteophyte formation in the neural
foramen. Instrument-scatter reduction software provides better resolution when
metallic artifact is of concern.
4.1.3 Myelography
is the injection of radiopaque material into the spinal subarachnoid space,
with x-rays then taken to define anatomy. It may be used as a pre-surgical
diagnostic procedure to obtain accurate information of characteristics,
location, and spatial relationships among soft tissue and bony structures. The
use of small needles and a less toxic, water-soluble, nonionic contrast is
recommended.
4.1.4 CT
Myelogram provides more detailed information about relationships between
neural elements and surrounding anatomy.
4.1.5 Lineal
Tomography is infrequently used, yet may be helpful in the evaluation of
bone surfaces, bony fusion, or pseudoarthrosis.
4.1.6 Bone
Scan (Radioisotope Bone Scanning) is generally accepted, well established,
and widely used. Bone scanning is more sensitive but less specific than MRI.
99mTechnetium diphosphonate uptake reflects osteoblastic activity and may be
useful in diagnosing metastatic/primary bone tumors, stress fractures,
osteomyelitis, and inflammatory lesions, but cannot distinguish between these
entities.
4.1.7 Other
Radioisotope Scanning: Indium and gallium scans are generally accepted,
well-established, and widely used procedures usually to help diagnose lesions
seen on other diagnostic imaging studies. 67Gallium citrate scans are used to
localize tumor, infection, and abscesses. 111Indium-labeled leukocyte scanning
is utilized for localizing infection or inflammation.
4.1.8 Dynamic
[Digital] Fluoroscopy: Dynamic [Digital] Fluoroscopy of the lumbar spine
measures the motion of intervertebral segments using a videofluoroscopy unit to
capture images as the subject performs lumbar flexion and extension, storing
the anatomic motion of the spine in a computer. Currently it is not recommended
for use in the diagnosis of lumbar instability, since there is limited information
on normal segmental motion for the age groups commonly presenting with low back
pain, and diagnostic criteria for specific spinal conditions are not yet
defined. No studies have yet demonstrated predictive value in terms of standard
operative and non-operative therapeutic outcomes.
4.2 OTHER
TESTS The following diagnostic procedures in this subsection are listed in
alphabetical order, not by importance:
4.2.1 Electrodiagnostic
Testing:
4.2.1.1 Electromyography
(EMG), Nerve Conduction Studies (NCS) These are generally accepted,
well-established and widely used diagnostic procedures. EMG and NCS, when
performed and interpreted by a trained physician/electrophysiologist, may be
useful for patients with suspected neural involvement whose symptoms are persistent
or unresponsive to initial conservative treatments. They are used to
differentiate peripheral neural deficits from radicular and spinal cord neural
deficits and to rule out concomitant myopathy. However, F-Wave Latencies are
not diagnostic for radiculopathy.
In general, EMG and NCS are complementary to
imaging procedures such as CT, MRI, and/or myelography or diagnostic injection
procedures. Electrodiagnostic studies may provide useful, correlative
neuropathophysiological information that would be otherwise unobtainable from
the radiologic studies discussed above.
4.2.1.2 Portable
Automated Electrodiagnostic Device (also known as Surface EMG) is not a
substitute for conventional diagnostic testing in clinical decision-making, and
therefore, is not recommended.
4.2.1.3 Somatosensory
Evoked Potential (SSEP) is not recommended to identify radiculopathy. It may be
used to evaluate myelopathy and other rare neurological disorders such as
neurogenic bladder and sexual dysfunction.
4.2.1.4 Current
Perception Threshold (CPT) Evaluation may be useful as a screening tool, but
its diagnostic efficacy in the evaluation of industrial low back pain has not
been determined. Therefore, CPT is not recommended as a diagnostic tool.
4.2.1.5 Large
Array Surface Electromyography measures low back muscle activity using a fixed
array of 63 electrodes arranged in 9 rows and 7 columns between the seventh
thoracic spinous process and the iliac crest. The array simultaneously collects
myoelectric data from multifidus, iliocostalis, quadratus lumborum, and other
lumbar muscles, which is analyzed for patterns of activity in these muscle
groups. It is used in researching physiologic changes and adaptations to back
pain, but is not recommended as a diagnostic procedure for individuals with
back pain due to a lack of interpretive standards.
4.2.1.6 Surface
EMG in combination with Range
of Motion and/or
Functional Capacity Evaluation This is designed to detect differences between
persons with and without low back pain, measuring signals in lumbar flexion
which show that painful paraspinal muscles fail to relax fully. It may show
aspects of the pathophysiology of muscle activity which advance the scientific
understanding of low back pain. The test also purports to determine the
significance of disc pathology and the age of an injury. It has not been
evaluated in a setting which tests a spectrum of patients commonly seen in
clinical practice, using an interpretation which is tested against a diagnostic
reference standard. Therefore, it is not
suitable as a diagnostic test for low back
pain and its use for this purpose is not recommended.
4.2.2 Injections — Diagnostic
4.2.2.1 Description
- Diagnostic spinal injections are generally accepted, well-established
procedures. These injections may be useful for localizing the source of pain,
and may have added therapeutic value when combined with injection of
therapeutic medication(s).
4.2.2.2 Indications
- Since these procedures are invasive, less invasive or non-invasive procedures
should be considered first. Selection of patients, choice of procedure, and
localization of the level for injection should be determined by clinical
information.
4.2.2.3 The
interpretation of the test results are primarily based on functional change,
symptom report, and pain response (via a recognized pain scale), before and at
an appropriate time period after the injection. The diagnostic significance of
the test result should be evaluated in conjunction with clinical information
and the results of other diagnostic procedures. Injections with local
anesthetics of differing duration may be used to support a diagnosis. In some
cases, injections at multiple levels may be required to accurately diagnose low
back pain.
Multiple injections provided at the same
session without staging may seriously dilute the diagnostic value of these
procedures. Practitioners must carefully weigh the diagnostic value of the
procedure against the possible therapeutic value.
4.2.2.4 Special
Requirements for Diagnostic Injections Since multi-planar fluoroscopy during
procedures is required to document technique and needle placement, an
experienced physician should perform the procedure. Permanent images are
required to verify needle placement. The subspecialty disciplines of the
physicians performing the injections may be varied, including, but not limited
to: anesthesiology, radiology, surgery, or physiatry. The practitioner should
document hands-on training through workshops of the type offered by
organizations such as the International Spine Intervention Society (ISIS)
and/or completed fellowship training with interventional training. They must
also be knowledgeable in radiation safety.
4.2.2.5 Specific
Diagnostic Injections In general, relief should last for at least the duration
of the local anesthetic used and should significantly relieve pain and result
in functional improvement. Refer to “Injections – Therapeutic” for information
on specific therapeutic injections.
4.2.2.5.1 Medial
Branch Blocks are generally accepted diagnostic injections, used to determine
whether a patient is a candidate for radiofrequency medial branch neurotomy
(also known as facet rhizotomy). To be a positive diagnostic block, the patient
should report a reduction of pain of 50% or greater relief from baseline or the
length of time appropriate for the local anesthetic used. A separate
comparative block on a different date may be performed to confirm the level of
involvement. A comparative block uses anesthetics of varying lengths of
activity.
Frequency and Maximum Duration: May be
repeated once for comparative blocks. Limited to 4 levels
4.2.2.5.2 Transforaminal
injections are generally accepted and useful in identifying spinal pathology.
When performed for diagnosis, small amounts of local anesthetic up to a total
volume of 1.0 cc should be used to determine the level of nerve root
irritation. A positive diagnostic block should result in a positive diagnostic
functional benefit and an 50% reduction in nerve-root generated pain
appropriate for the anesthetic used as measured by accepted pain scales (such
as a VAS).
Frequency and Maximum Duration: Once per
suspected level. Limited to three levels. May be repeated once for
confirmation.
4.2.2.5.3 Zygapophyseal
(Facet) Blocks: Facet blocks are generally accepted. They may be used
diagnostically to direct functional rehabilitation programs. A positive
diagnostic block should result in a positive diagnostic functional benefit and
an 50% reduction in pain appropriate for the anesthetic used as measured by
accepted pain scales (such as a VAS). They then
may be repeated per the therapeutic
guidelines. Frequency and maximum Duration: Once per suspected level, limited
to three levels. May be repeated for confirmation.
4.2.2.5.4 Sacroiliac
Joint Injection:
4.2.2.5.4.1 Description
- A generally accepted Injection of local anesthetic in an intra-articular
fashion into the sacroiliac joint under fluoroscopic guidance. Long-term
therapeutic effect has not yet been established.
4.2.2.5.4.2 Indications
- Primarily diagnostic to rule out sacroiliac joint dysfunction versus other pain
generators. Intra-articular injection can be of value in diagnosing the pain
generator. There should be documented at least 50% pain relief (as measured by
accepted pain scales such as a VAS)
Frequency and Maximum Duration:
May be repeated for confirmation.
4.2.3 Provocation
Discography:
4.2.3.1 Description
- Discography is an accepted diagnostic procedure to identify or refute a
discogenic source of pain for patients who are surgical candidates. Discography
should only be performed by physicians who are experienced and have been
proctored in the technique. It is essential that all indications,
pre-conditions, special considerations, procedures, reporting requirements, and
results are carefully and specifically followed. Results should be interpreted
judiciously.
4.2.3.2 Indications
- Discography may be indicated when a patient has a history of functionally
limiting, unremitting low back pain of greater than four months duration, with
or without leg pain, which has been unresponsive to all conservative
interventions. A patient who would not consider operative therapeutic
intervention is not a candidate for an invasive non-therapeutic intervention,
such as provocation discography.
Discography may prove useful for the
evaluation of the pre-surgical spine, such as
pseudarthrosis, discogenic pain at levels
above or below a prior spinal fusion, annular
tear, or internal disc disruption.
Discography may show disc degeneration and
annular disruption in the absence of low back pain. Discography may also elicit
concordant pain in patients with mild and functionally inconsequential back
pain. Because patients with mild back pain should not be considered for
invasive treatment, discography should not be performed on these patients. In
symptomatic patients with annular tears on discography, the side of the tear
does not necessarily correlate with the side on which the symptoms occur. The
presence of an annular tear does not necessarily identify the tear as the pain
generator.
Discography may have a limited place in the
work-up of pseudarthrosis. Discography may prove useful in evaluating the
number of lumbar spine levels that might require fusion. CT-Discography
provides further detailed information about morphological abnormalities of the
disc and possible lateral disc herniations.
4.2.3.3 Pre-conditions
for provocation discography include all of the following:
4.2.3.3.1 A
patient with functionally limiting, unremitting back and/or leg pain of greater
than four months duration in whom conservative treatment has been unsuccessful
and in whom the specific diagnosis of the pain generator has not been made
apparent on the basis of other noninvasive imaging studies (e.g., MRI, CT,
plain films, etc.). It is recommended that discography be reserved for use in
patients with equivocal MRI findings, especially at levels adjacent to clearly
pathological levels. Discography may be more sensitive than MRI or CT in
detecting radial annular tears. However, radial tears must always be correlated
with clinical presentation.
4.2.3.3.2 Patients
who are considered surgical candidates (e.g., symptoms are of sufficient
magnitude and the patient has been informed of the possible surgical options
that may be available based upon the results of discography).
4.2.3.3.3 Informed
consent regarding the risks and potential diagnostic benefits of discography
has been obtained.
4.2.3.4 Special
Considerations:
4.2.3.4.1 Discography
should not be performed by the physician expected to perform the therapeutic
procedure. The procedure should be carried out by an experienced individual who
has received specialized training in the technique of provocation discography.
4.2.3.4.2 Discography
should be performed in a blinded format that avoids leading the patient with
anticipated responses. The procedure should include one or more disc levels
thought to be normal or non-painful in order to serve as an internal control.
The patient should not know what level is being injected in order to avoid
spurious results. Abnormal disc levels may be repeated to confirm concordance.
4.2.3.4.3 Sterile
technique must be utilized.
4.2.3.4.4 Judicious
use of light sedation during the procedure is acceptable, represents the most
common practice nationally at the current time, and is recommended by most
experts in the field. The patient must be awake and able to accurately report
pain levels during the provocation portion of the procedure.
4.2.3.4.5 The
discography should be performed using a manometer to record pressure.
4.2.3.4.6 Intradiscal
injection of local anesthetic may be carried out after the provocation portion
of the examination and the patient’s response.
4.2.3.4.7 It
is recommended that a post-discogram CT be considered as it frequently provides
additional useful information about disc morphology or other pathology.
4.2.3.5 Reporting
of Discography - In addition to a narrative report, the discography report
should contain a standardized classification of (a) disc morphology (b) the
pain response, and (c) the pressure at which pain is produced. All results
should be clearly separated in the report from the narrative portion.
Asymptomatic annular tears are common and the concordant pain response is an
essential finding for a positive discogram.
When discography is performed to identify
the source of a patient’s low-back pain, both a concordant pain response and
morphological abnormalities must be present at the pathological level prior to
initiating any treatment directed at that level. The patient must be awake
during the provocation phase of the procedure; therefore, sedative medication
must be carefully titrated.
4.2.3.5.1 Reporting
disc morphology as visualized by the post-injection CT scan (when available)
should follow the Modified Dallas Discogram Scale where:
·
Grade 0 = Normal Nucleus
·
Grade 1 = Annular
tear confined to inner one-third of annulus fibrosis.
·
Grade 2 = Annular
tear extending to the middle third of the annulus fibrosis.
·
Grade 3 = Annular
tear extending to the outer one-third of the annulus fibrosis.
·
Grade 4 = A grade
3 tear plus dissection within the outer annulus to involve more than 30° of the
disc circumference.
·
Grade 5 = Full
thickness tear with extra-annular leakage of contrast, either focal or diffuse.
4.2.3.5.2 Reporting of pain response should be
consistent with the operational criteria of the International Spine
Intervention Society (ISIS) Guidelines. The report must include the level of
concordance for back pain and leg pain separately using a 10-point VAS, or
similar quantitative assessment. It should be noted that change in the VAS
scale before and after provocation is more important than the number reported.
4.2.3.5.2.1 Unequivocal
Discogenic Pain
·
Stimulation of
the target disc reproduces concordant pain
·
The pain is
registered as at least 6 on a 10-point VAS.
·
The pain is
reproduced at a pressure of less than 15 psi above opening pressure; and
·
Stimulation of
two adjacent discs does not produce pain at all
4.2.3.5.2.2 Definite
Discogenic Pain
·
Stimulation of
the target disc reproduces concordant pain
·
The pain is
registered as at least 6 on a 10-point VAS.
·
The pain is
reproduced at a pressure of less than 15 psi above opening pressure; and
·
Stimulation of at
least one adjacent disc does not produce pain at all
4.2.3.5.2.3 Highly
Probable Discogenic Pain
·
Stimulation of
the target disc reproduces concordant pain
·
That pain is
registered as at least 6 on a 10-point VAS.
·
That the pain is
reproduced at a pressure of less than 50 psi above opening pressure; and
·
Stimulation of
two adjacent discs does not produce pain at all
4.2.3.5.2.4 Probable
Discogenic Pain
·
Stimulation of
the target disc reproduces concordant pain
·
That pain is
registered as at least 6 on a 10-point VAS.
·
The pain is
reproduced at a pressure of less than 50 psi above opening pressure; and
·
Stimulation of
one adjacent disc does not produce pain at all, and stimulation of another
adjacent discs at greater than 50 psi, produces pain, but the pain is not
concordant.
Multiple combinations of factors are possible.
However, if the patient does not qualify for at least a ‘Probable Discogenic
Pain’ level, then the discogram should probably be considered negative. The VAS
score prior to the discogram should be taken into account when interpreting the
VAS score reported by the patient during the discogram.
4.2.4 Thermography: is an accepted and established
procedure, but has no use as a diagnostic test for low back pain and is not
recommended.
Patients undergoing therapeutic procedure(s)
are encouraged to return to modified or restricted duty during their
rehabilitation at the earliest appropriate time. Refer to “Return-to-Work” in
this section for detailed information.
Cessation and/or review of treatment
modalities should be undertaken when no further significant subjective or
objective improvement in the patient’s condition is noted. If patients are not
responding within the recommended duration periods, alternative treatment
interventions, further diagnostic studies or consultations should be pursued.
Providers should provide and document education to the patient. No treatment
plan is complete without addressing issues of individual and/or group patient
education as a means of facilitating self-management of symptoms.
Home therapy is an important component of
therapy and may include active and passive therapeutic procedures, as well as,
other modalities to assist in alleviating pain, swelling, and abnormal muscle
tone.
The following procedures are listed in
alphabetical order.
5.1 ACUPUNCTURE
is an accepted and widely used procedure for the relief of pain and inflammation. The exact mode of action is only partially understood. Western medicine studies suggest that acupuncture stimulates the nervous system at the level of the brain, promotes deep relaxation, and affects the release of neurotransmitters. Acupuncture is commonly used as an alternative or in addition to traditional Western pharmaceuticals. While it is commonly used when pain medication is reduced or not tolerated, it may be used as an adjunct to physical rehabilitation and/or surgical intervention to hasten the return of functional activity. Acupuncture should be performed by MD, DO, DC with appropriate training; or a licensed acupuncturist.
5.1.1 Acupuncture:
is the insertion and removal of filiform needles to stimulate acupoints (acupuncture
points). Needles may be inserted, manipulated, and retained for a period of
time. Acupuncture can be used to reduce pain, reduce inflammation, increase
blood flow, increase range of motion, decrease the side effect of
medication-induced nausea, promote relaxation in an anxious patient, and reduce
muscle spasm.
Indications include joint pain, joint
stiffness, soft tissue pain and inflammation, paresthesia, postsurgical pain
relief, muscle spasm, and scar tissue pain.
5.1.2 Acupuncture
with Electrical Stimulation: is the use of electrical current
(micro-amperage or milli-amperage) on the needles at the acupuncture site. It
is used to increase effectiveness of the needles by continuous stimulation of
the acupoint. Physiological effects (depending on location and settings) can
include endorphin release for pain relief, reduction of inflammation, increased
blood circulation, analgesia through interruption of pain stimulus, and muscle
relaxation.
It is indicated to treat chronic pain
conditions, radiating pain along a nerve pathway, muscle spasm, inflammation,
scar tissue pain, and pain located in multiple sites.
5.1.3 Total
Time Frames For Acupuncture and Acupuncture with Electrical Stimulation:
Time frames are not meant to be applied to each of the above sections
separately. The time frames are to be applied to all acupuncture treatments
regardless of the type or combination of therapies being provided.
Time to produce effect: 3 to 6 treatments
Frequency: 1 to 3 times per week Maximum course
duration:
14 treatments (one course) Any of the above acupuncture treatments may extend
longer if objective functional gains can be documented or when symptomatic
benefits facilitate progression in the patient’s treatment program. An
additional course of treatment beyond 14 treatments may be documented with
respect to need and ability to facilitate positive symptomatic or functional
gains. Such care should be re-evaluated and documented with each series of
treatments.
5.1.4 Other
Acupuncture Modalities: Acupuncture treatment is based on individual
patient needs and therefore treatment may include a combination of procedures
to enhance treatment effect. Other procedures may include the use of heat, soft
tissue manipulation/massage, and exercise. Refer to Active Therapy (Therapeutic
Exercise) and Passive Therapy sections (Massage and Superficial Heat and Cold
Therapy) for a description of these adjunctive acupuncture modalities and time
frames.
5.2 BIOFEEDBACK
is a form of behavioral medicine that helps patients learn self-awareness
and self-regulation skills for the purpose of gaining greater control of their
physiology, such as muscle activity, brain waves, and measures of autonomic
nervous system activity. Electronic instrumentation is used to monitor the targeted
physiology and then displayed or fed back to the patient visually,
auditorially, or tactilely, with coaching by a biofeedback specialist.
Biofeedback is provided by clinicians certified in biofeedback and/or who have
documented specialized education, advanced training, or direct or supervised
experience qualifying them to provide the specialized treatment needed (e.g.,
surface EMG, EEG, or other).
Treatment is individualized to the patient’s
work-related diagnosis and needs. Home practice of skills is required for
mastery and may be facilitated by the use of home training tapes. The ultimate
goal of biofeedback treatment is to normalize physiology to the pre-injury
status to the extent possible, and involves transfer of learned skills to the
workplace and daily life. Candidates for biofeedback therapy or training must
be motivated to learn and practice biofeedback and self-regulation techniques.
Indications for biofeedback include individuals who are suffering from musculoskeletal
injury in which muscle dysfunction or other physiological indicators of
excessive or prolonged stress response affects and/or delays recovery. Other
applications include training to improve self-management of emotional
stress/pain responses such as anxiety, depression, anger, sleep disturbance,
and other central and autonomic nervous system imbalances. Biofeedback is often
used in conjunction with other treatment modalities.
Time
to produce effect: 3 to 4 visits Frequency: 1 to 2 times per week Maximum
duration: 10 to 12 visits. Treatment beyond 12 visits must be documented with
respect to
need, expectation, and ability to facilitate
positive functional gains.
5.3 INJECTIONS
— THERAPEUTIC
5.3.1 Therapeutic
Spinal Injections: Description - Therapeutic spinal injections may be used
after initial conservative treatments have
been
undertaken. Therapeutic injections should be used only after imaging studies
and/or diagnostic injections have established pathology. Special Considerations
- For all injections (excluding trigger point), multi-planar fluoroscopic
guidance during procedures is required to
document technique and needle placement, and should be performed by a physician
experienced in the procedure. Permanent images are required to verify needle
replacement. The subspecialty disciplines of the physicians performing
injections may be varied, including, but not limited to: anesthesiology,
radiology, surgery, or physiatry. The practitioner should document hands-on
training through workshops of the type offered by organizations such as the
International Spine Intervention Society (ISIS) and/or completed fellowship
training in pain medicine with interventional training. They must also be
knowledgeable in radiation safety.
5.3.1.1 Epidural
Steroid Injection (ESI)
5.3.1.1.1 Description
- Epidural steroid injections are injections of corticosteroid into the
epidural space. The purpose of ESI is to reduce pain and inflammation in the
acute or sub-acute phases of injury. ESI uses three approaches: transforaminal,
interlaminar (midline), and caudal.
5.3.1.1.2 Needle
Placement - Multi-planar fluoroscopic imaging is required for all epidural
steroid injections. Contrast epidurograms allow one to verify the flow of
medication into the epidural space. Permanent images are required to verify
needle replacement.
5.3.1.1.3 Indications
- There is some evidence that epidural steroid injections are effective for
patients with radicular pain or radiculopathy (sensory or motor loss in a
specific dermatome or myotome). Up to 80% of patients with radicular pain may
have initial relief. However, only 25-57% are likely to have excellent
long-term relief. Although there is no evidence regarding the effectiveness of
ESI for non-radicular disc herniation, it is an accepted intervention.
Frequency: One or more levels can be
injected in one session. Whether injections are repeated depends upon the
patient’s response to the previous injection. Subsequent injections may occur.
Injections can be repeated if the patient has demonstrated functional gain
and/or pain returns or worsens.
Maximum duration: Six treatments (a
treatment may include injections at one or two levels) may be done in one year,
as per the patient’s response to pain and function. Patients should be
reassessed for improvement in pain (as measured by accepted pain scales) and/or
evidence of functional improvement.
5.3.1.2 Zygapophyseal
(Facet) Injection
5.3.1.2.1 Description
- A generally accepted intra-articular or pericapsular injection of local
anesthetic and corticosteroid.
5.3.1.2.2 Indications-
Patients with pain suspected to be facet mediated in origin. In these
patients, facet injections may be occasionally useful in facilitating
rehabilitation Facet injections may be repeated if they result in increased
documented functional benefit for at least 4 to 6 weeks and/or at least an 50%
initial improvement in pain scales as measured by accepted pain scales (such as
VAS).
Maximum Duration: 4 per level per year. Maximum three levels
5.3.1.2.3 Sacroiliac
Joint Injection:
5.3.1.2.3.1 Description
- A generally accepted injection of local anesthetic in an intra-articular
fashion into the sacroiliac joint under radiographic guidance. May include the
use of corticosteroids. Long-term therapeutic effect has not yet been
established.
5.3.1.2.3.2 Indications
- Primarily diagnostic to rule out sacroiliac joint dysfunction vs. other pain
generators. Intra-articular injection can be of value in diagnosing the pain
generator. These injections may be repeated if they result in increased
documented functional benefit for at least 6 weeks and/or at least an 50%
initial improvement in pain scales as measured by accepted pain scales (such as
VAS). Maximum duration: 4 injections per year.
5.3.1.2.4 Intradiscal
Steroid Therapy: Intradiscal Steroid Therapy consists of injection of a steroid
preparation into the intervertebral disc under fluoroscopic guidance at the
time of discography. There is good evidence that it is not effective in the
treatment of suspected discogenic back pain and its use is not recommended.
5.3.2 Radio Frequency
Medial Branch Neurotomy/facet rhizotomy:
5.3.2.1 Description
-A procedure designed to denervate the facet joint by ablating the
corresponding sensory medial branches. Continuous percutaneous radiofrequency
is the method generally used.
There is good evidence to support Radio Frequency Medial Branch
Neurotomy in the cervical spine but benefits beyond one year are not yet
established. Evidence in the lumbar spine is conflicting; however, the
procedure is generally accepted. In one study, 60% of patients maintained at
least 90% pain relief at 12 months. Radio-frequency Medial Branch Neurotomy is
the procedure of choice over alcohol, phenol, or cryoablation. Precise
positioning of the probe using fluoroscopic guidance is required. Permanent
images should be recorded to verify placement of the device.
5.3.2.2 Indications
- Those patients with significant, facetogenic pain. Individuals should have
met all of the following indications: Pain of well-documented facet origin,
unresponsive to active and/or passive therapy. It is generally recommended that
this procedure not be performed until three months of conservative therapy have
been completed. All patients should continue appropriate exercise with
functionally directed rehabilitation. Active treatment, which patients will
have had prior to the procedure, will frequently require a repeat of the
sessions previously ordered. All patients should have a successful response to
a diagnostic medial nerve branch block and a separate comparative block. To be
a positive diagnostic block the patient should report a reduction of pain of
50% or greater from baseline for the length of time appropriate for the local
anesthetic used. It is suggested that this be recorded on a form. A separate
comparative
block on a different date may be performed to confirm the level of
involvement. A comparative block uses anesthetics with varying lengths of
activity.
5.3.2.3 Requirements
for Repeat Radiofrequency Medial Branch Neurotomy (or additional-level RF
Neurotomy): In some cases pain may recur. Successful RF Neurotomy usually
provides from six to eighteen months of relief.
Before a repeat RF Neurotomy is done, a
confirmatory medial branch injection should be performed if the patient’s pain
pattern presents differently than the initial evaluation. In occasional
patients, additional levels of RF neurotomy may be necessary. The same
indications and limitations apply.
5.3.3 Sacro-iliac
(SI) Joint Radiofrequency Denervation: is a denervation of the SI joint.
This procedure is not recommended.
5.3.4 Trigger
Point Injections and Dry Needling Treatment:
5.3.4.1 Description
-Trigger point injections are a generally accepted treatment. Trigger point
treatment can consist of dry needling or injection of local anesthetic, with or
without corticosteroid, into highly localized, extremely sensitive bands of
skeletal muscle fibers that produce local and referred pain when activated.
Injection efficacy may be enhanced if injections are immediately followed by
myofascial therapeutic interventions, such as vapocoolant spray and stretch,
ischemic pressure massage (myotherapy), specific soft tissue mobilization and
physical modalities. A truly blinded study comparing dry needle treatment of
trigger points is not feasible. There is no evidence that injection of
medications improves the results of trigger-point injections. Needling alone
may account for some of the therapeutic response.
There is no indication for conscious
sedation for patients receiving trigger point injections. The patient must be
alert to help identify the site of the injection.
5.3.4.2 Indications
- Trigger point injections may be used to relieve myofascial pain and
facilitate active therapy and stretching of the affected areas. They are to be
used as an adjunctive treatment in combination with other treatment modalities
such as functional restoration programs. Trigger point injections should be
utilized primarily for the purpose of facilitating functional progress.
Patients should continue in therapeutic exercise program as tolerated
throughout the time period they are undergoing intensive myofascial
interventions. Myofascial pain is often associated with other underlying
structural problems and any abnormalities need to be ruled out prior to
injection.
Trigger point injections are indicated in
those patients where well circumscribed trigger points have been consistently
observed Generally, these injections are not necessary unless consistently
observed trigger points are not responding to specific, noninvasive, myofascial
interventions within approximately a 6-week time frame. However, trigger point
injections may be occasionally effective when utilized in the patient with
immediate, acute onset of low back pain.
Frequency: Weekly. Suggest no more than 4
injection sites per session per week to avoid
significant post-injection soreness.
Maximum duration: 8 weeks. Occasional patients may require 2 to 4 repetitions
over a 1 to
2 year period.
5.3.5 Prolotherapy:
also known as sclerotherapy consists of a series of injections of
hypertonic dextrose, with or without glycerine and phenol, into the ligamentous
structures of the low back. Its proponents claim that the inflammatory response
to the injections will recruit cytokine growth factors involved in the proliferation
of connective tissue, stabilizing the ligaments of the low back when these
structures have been damaged by mechanical insults.
There are conflicting studies concerning the
effectiveness of Prolotherapy in the low back. Lasting functional improvement
has not been shown. The injections are invasive, may be painful to the patient,
and are not generally accepted or widely used. Therefore, the use of
Prolotherapy for low back pain is not recommended.
5.3.6 Epiduroscopy
and Epidural Lysis of Adhesions: is an investigational treatment of low
back pain. It involves the introduction of a fiberoptic endoscope into the
epidural space via the sacral hiatus. With cephalad advancement of the
endoscope under direct visualization, the epidural space is irrigated with
saline. Adhesiolysis may be done mechanically with a fiberoptic endoscope. The
saline irrigation is performed with or without epiduroscopy and is intended to
distend the epidural space in order to obtain an adequate visual field. It is
designed to produce lysis of adhesions, which are conjectured to produce
symptoms due to traction on painful nerve roots. Saline irrigation is
associated with risks of elevated pressures which may impede blood flow and
venous return, possibly causing ischemia of the cauda equina and retinal
hemorrhage.
Other complications associated with
instrumented lysis include catheter shearing, need for catheter surgical
removal, infection (including meningitis), hematoma, and possible severe hemodynamic
instability during application. Although epidural adhesions have been
postulated to cause chronic low back pain, studies have failed to find a
significant correlation between the level of fibrosis and pain or difficulty
functioning. Studies of epidural lysis demonstrate no transient pain relief
from the procedure. Given the low likelihood of a positive response, the
additional costs and time requirement, and the possible complications from the
procedure, epidural injection, or mechanical lysis, is not recommended.
Epiduroscopy-directed steroid injections are
also not recommended as there is no evidence to support an advantage for using
an epiduroscope with steroid injections.
5.4 MEDICATIONS
use in the treatment of low back injuries is appropriate for controlling acute
and chronic pain and inflammation. Use of medications will vary widely due to
the spectrum of injuries from simple strains to post-surgical healing. All
drugs should be used according to patient needs. A thorough medication history,
including use of alternative and over the counter medications, should be
performed at the time of the initial visit and updated periodically. The
patient should be educated regarding the interaction with prescription and
over-the-counter medications as well as the contents of over-the-counter herbal
products.
The
use of generic medications is encouraged. The list below is not all inclusive.
It is accepted that
medications not on this list may be appropriate for use in the care of the
injured worker.
The following are listed in alphabetical order:
5.4.1 Acetaminophen:
is an effective analgesic with antipyretic but not anti-inflammatory
activity. Acetaminophen is generally well tolerated, causes little or no
gastrointestinal irritation, and is not associated with ulcer formation.
Acetaminophen has been associated with liver toxicity in overdose situations or
in chronic alcohol use. Patients may not realize that many over-the-counter
preparations may contain acetaminophen.
The total daily dose of acetaminophen is recommended not to exceed 4
grams per 24-hour period, from all sources, including narcotic-acetaminophen
combination preparations.
5.4.2 Muscle
Relaxants: are appropriate for muscle spasm with pain. There is strong
evidence that muscle relaxants are more effective than placebo for providing
short-term pain relief in acute low back pain. When prescribing these agents,
physicians must seriously consider side effects of drowsiness or dizziness and
the fact that benzodiazepines may be habit-forming
5.4.3 Narcotics:
should be primarily reserved for the treatment of severe low back pain. In mild
to moderate cases of low back pain, narcotic medication should be used
cautiously on a case-bycase basis. Adverse effects include respiratory
depression, the development of physical, and impaired alertness.
Narcotic medications should be prescribed
with strict time, quantity, and duration guidelines, and with definitive
cessation parameters. Pain is subjective in nature and should be evaluated
using a scale to rate effectiveness of the narcotic prescribed.
5.4.4 Nonsteroidal
Anti-Inflammatory Drugs (NSAIDs): are useful for pain and inflammation. In
mild cases, they may be the only drugs required for analgesia. There are
several classes of NSAIDs, and the response of the individual injured worker to
a specific medication is unpredictable. For this reason, a range of NSAIDs may
be tried in each case with the most effective preparation being continued.
Patients should be closely monitored for adverse reactions. Naproxen sodium
does not appear to be associated with increased risk of vascular events.
Administration of proton pump inhibitors, Histamine 2 Blockers or prostaglandin
analog misoprostol along with these NSAIDs may reduce the risk of duodenal and
gastric ulceration. Intervals for metabolic screening are dependent upon the
patient's age, general health status and should be within parameters listed for
each specific medication. Complete Blood Count (CBC), and liver and renal
function should be monitored in patients on chronic NSAIDs and initially when
indicated.
5.4.4.1 Selective
Cyclo-oxygenase-2 (COX-2) Inhibitors COX-2 inhibitors are more recent NSAIDs
and differ in adverse side effect profiles from
the traditional NSAIDs. The major advantages of selective COX-2
inhibitors over traditional NSAIDs are that they have less gastrointestinal
toxicity and no platelet effects. COX-2 inhibitors should not be first-line for
low risk patients who will be using an NSAID
short term but are indicated in select patients for whom traditional
NSAIDs are not tolerated. Serious upper GI adverse events can occur even in
asymptomatic patients. Patients at high risk for GI bleed include those who use
alcohol, smoke, are older than 65, take corticosteroids or anti-coagulants, or
have a longer duration of therapy.
5.4.5 Psychotropic/Anti-anxiety/Hypnotic
Agents: may be useful for treatment of mild and chronic pain, dysesthesias,
sleep disorders, and depression. Antidepressant medications, such as tricyclics
and Selective Serotonin Reuptake Inhibitors (SSRIs), are useful for affective
disorder and chronic pain management. Tricyclic antidepressant agents, in low
dose, are useful for chronic pain.
Anti-anxiety
medications should generally be limited to short-term use. Combinations of the
above
agents
may be useful.
As a general rule, physicians should access the patient’s prior history of
substance abuse or
depression prior to prescribing any of these agents. Due to the habit-forming
potential of the
benzodiazepines and other drugs found in this class, they are not routinely
recommended.
5.4.6 Tramadol:
is useful in relief of low back pain and has been shown to provide pain relief
equivalent to that of commonly prescribed NSAIDs. Although Tramadol may cause
impaired alertness, it is generally well tolerated, does not cause
gastrointestinal ulceration, or exacerbate hypertension or congestive heart
failure.
5.5 OCCUPATIONAL
REHABILITATION PROGRAMS
5.5.1 Non-Interdisciplinary:
These generally accepted programs are work-related, outcome-focused,
individualized treatment programs. Objectives of the program include, but are
not limited to, improvement of cardiopulmonary and neuromusculoskeletal
functions (strength, endurance, movement, flexibility, stability, and motor
control functions), patient education, and symptom relief. The goal is for
patients to gain full or optimal function and return to work. The service may
include the time-limited use of passive modalities with progression to active
treatment and/or simulated/ real work.
5.5.1.1 Work
Conditioning/Simulation This program may begin once a patient is out of the
acute
phase of injury and will be able to tolerate this program. These
programs are usually initiated after the acute phase has been completed and
offered at any time throughout the recovery phase. Work conditioning should be
initiated when imminent return of a patient to modified or full duty is not an
option, but the prognosis for returning the patient to work at completion of
the program is at least fair to good. The need for work place simulation should
be based upon the results of a Functional Capacity Evaluation and/or Jobsite
Analysis.
Length of visit: 1 to 4 hours per day. Frequency: 2 to 5 visits per week
Maximum duration: 8 weeks. Participation in a program beyond six weeks must be
documented with respect to need and the ability to facilitate positive
symptomatic or
functional gains.
5.5.1.2 Work
Hardening Work Hardening is an interdisciplinary program addressing a patient’s
employability and return to work. It includes a progressive increase in the
number of hours per day that a patient completes work simulation tasks until
the patient can tolerate a full workday. This is
accomplished by addressing the medical, behavioral, physical,
functional, and vocational components of employability and return-to-work.
This can include a highly structured program
involving a team approach or can involve any of the components thereof. The
interdisciplinary team should, at a minimum, be comprised of a qualified
medical director who is board certified with documented training in
occupational rehabilitation; team physicians having experience in occupational
rehabilitation; occupational therapist; physical therapist; case manager; and
psychologist. As appropriate, the team may also include: chiropractor, RN,
vocational specialist or Certified Biofeedback Therapist.
Length of visit: Up to 8 hours/day
Frequency: 2 to 5 visits per week Maximum duration: 8 weeks. Participation in a
program beyond six weeks must be
documented with respect to need and the
ability to facilitate positive symptomatic or functional gains.
5.5.1.3 Spinal
Cord Programs Spinal Cord Systems of Care provide coordinated, case-managed,
and integrated service for people with spinal cord dysfunction, whether due to
trauma or disease. The system includes an inpatient component in an
organization licensed as a hospital and an outpatient component. Each component
endorses the active participation and choice of the persons served throughout
the entire program. The Spinal Cord System of Care also
provides or formally links with key
components of care that address the lifelong needs of
the persons served.
This can include a highly structured program
involving a team approach or can involve any
of the components thereof. The
interdisciplinary team should, at a minimum, be comprised of a qualified
medical director who is board certified and trained in rehabilitation, a case
manager, occupational therapy, physical therapy, psychologist, rehabilitation
RN and MD, and therapeutic recreation specialist. As appropriate, the team may
also include: rehabilitation counselor, respiratory therapist, social worker,
or speech-language pathologist.
Time frame durations for any spinal cord
program should be determined based upon the extent of the patient’s injury and
at the discretion of the rehabilitation physician in charge.
5.6 ORTHOTICS
5.6.1 Foot
Orthoses and Inserts: are accepted interventions for spinal disorders that
are due to aggravated mechanical abnormalities, such as leg length discrepancy,
scoliosis, or lower extremity misalignment. Shoe insoles or inserts may be
effective for patients with acute low back problems who stand for prolonged
periods of time.
5.6.2 Lumbar
Support Devices: include backrests for chairs and car seats. Lumbar
supports may provide symptomatic relief of pain and movement reduction in cases
of chronic low back problems.
5.6.3 Lumbar
Corsets and Back Belts: The injured worker should be advised of the
potential harm from using a lumbar support for a period of time greater than
that which is prescribed. Harmful effects include de-conditioning of the trunk
musculature, skin irritation, and general discomfort.
5.6.4 Lumbosacral
Bracing: Rigid bracing devices are well accepted and commonly used for
post-fusion, scoliosis, and vertebral fractures.
5.7 PATIENT
EDUCATION No treatment plan is complete without addressing issues of
individual and/or group patient education as a means of prolonging the
beneficial effects of treatment, as well as facilitating self-management of
symptoms and injury prevention. The patient should be encouraged to take an
active role in the establishment of functional outcome goals. They should be
educated on their specific injury, assessment findings, and plan of treatment.
Instruction on proper body mechanics and posture, positions to avoid, self-care
for exacerbation of symptoms, and home exercise should also be addressed.
Time to produce effect: Varies with
individual patient
Frequency: Should occur at every visit.
5.8 RESTRICTION
OF ACTIVITIES Continuation of normal daily activities is the recommendation
for acute and chronic low back pain without neurologic symptoms. There is good
evidence against the use of bed rest in cases without neurologic symptoms. Bed
rest may lead to de-conditioning and impair rehabilitation. Modified
return-to-work is almost always more efficacious and rarely contraindicated in
the vast majority of injured workers with low back pain.
5.9 RETURN-TO-WORK
Early return-to-work should be a prime goal in treating occupational
injuries given the poor return-to-work prognosis for an injured worker who has
been out of work for more than six months. It is imperative that the patient be
educated regarding the benefits of return-to-work, work restrictions, and
follow-up if problems arise. When attempting to return a patient to work after
a specific injury, clear objective physical capabilities of the injured worker
should be outline on the appropriate form. An accurate job description with
detailed physical duty requirements is often necessary to assist the physician
in making return-to-work recommendations.
Employers should be prepared to offer
transitional work. This may consist of temporary work in a less demanding
position, return to the regular job with restrictions, or gradual return to the
regular job. Company policies which encourage return-to-work with positive communication
are most likely to have decreased worker disability. This may require a job
site evaluation. When an appropriate a Jobsite Analysis may be necessary.
Return-to-work is defined as any work or
duty that the patient is able to perform safely. It may not be the patient’s
regular work. Due to the large spectrum of injuries of varying severity and
varying physical demands in the work place, it is not possible to make specific
return-to-work guidelines for each injury.
Compliance with Activity Restrictions: In some cases, compliance with restriction
of activity levels may require a complete job site evaluation, a functional
capacity evaluation (FCE) or other special testing.
5.10 THERAPY
— PASSIVE Most of the following passive therapies and modalities are
generally accepted methods of care for a variety of work-related injuries.
Passive therapy includes those treatment modalities that do not require energy
expenditure on the part of the patient. They are principally effective during
the early phases of treatment and are directed at controlling symptoms such as
pain, inflammation and swelling and to improve the rate of healing soft tissue
injuries. They should be used adjunctively with active therapies such as
postural stabilization and exercise programs to help control swelling, pain,
and inflammation during the active rehabilitation process. Please refer to
General Guideline Principles, Active Interventions. Passive
therapies may be used intermittently as a therapist deems appropriate or
regularly if there are specific goals with objectively measured functional
improvements during treatment.
Generally, passive interventions are viewed
as a means to facilitate progress in an active rehabilitation program with
concomitant attainment of objective functional gains. All rehabilitation
programs must incorporate “Active Interventions” no later than twelve visits or
three weeks after the onset of treatment. Reimbursement for passive modalities
only after the first twelve visits or three weeks of treatment without clear
evidence of Active Interventions will require supportive documentation.
On occasion, specific diagnoses and
post-surgical conditions may warrant durations of treatment beyond those listed
as "maximum.” factors such as exacerbation of symptoms, re-injury,
interrupted continuity of care, and comorbitities may also extend durations of
care. Specific goals with objectively measured functional improvement during
treatment must be cited to justify extended durations of care. It is
recommended that, if no functional gain is observed after the number of
treatments under “time to produce effect” have been completed; alternative
treatment interventions, further diagnostic studies, or further consultations
should be pursued.
The following passive therapies are listed
in alphabetical order:
5.10.1 Electrical
Stimulation (Unattended and Attended): is an accepted treatment. Once
applied, unattended electrical stimulation requires minimal on-site supervision
by the provider. Indications include pain, inflammation, muscle spasm, atrophy,
decreased circulation, and the need for osteogenic stimulation. A home unit
should be purchased if treatment is effective and frequent use is recommended.
Time to produce effect: 2 to 4 treatments
Maximum
duration: 14 visits
5.10.2 Iontophoresis: is an
accepted treatment which consists of the transfer of medication, including, but
not limited to, steroidal anti-inflammatories and anesthetics, through the use
of electrical stimulation. Indications include pain (Lidocaine), inflammation
(hydrocortisone, salicylate), edema (mecholyl, hyaluronidase, salicylate),
ischemia (magnesium, mecholyl, iodine), muscle spasm (magnesium, calcium),
calcific deposits (acetate), scars, and keloids (sodium chloride, iodine,
acetate). There is no proven benefit for this therapy in the low back.
Time
to produce effect: 1 to 4 treatments
Frequency: 3 times per week with at least 48 hours between treatments
Maximum duration: 8 visits per body region
5.10.3 Manipulation: Is
generally accepted, well-established and widely used therapeutic intervention
for low back pain. Manipulative Treatment (not therapy) is defined as the
therapeutic application of manually guided forces by an operator to improve
physiologic function and/or support homeostasis that has been altered by the
injury or occupational disease, and has associated clinical significance.
High
velocity, low amplitude (HVLA) technique, chiropractic manipulation,
osteopathic
manipulation, muscle energy techniques, counter strain, and non-force
techniques are all types of
manipulative treatment. This may be applied by osteopathic physicians (D.O.),
chiropractors
(D.C.), properly trained physical therapists (P.T.), or properly trained
medical physicians. Under
these different types of manipulation exist many subsets of different
techniques that can be
described as a) direct- a forceful engagement of a restrictive/pathologic
barrier, b) indirect- a
gentle/non-forceful disengagement of a restrictive/pathologic barrier, c) the
patient actively assists
in the treatment and d) the patient relaxing, allowing the practitioner to move
the body tissues.
When the proper diagnosis is made and coupled with the appropriate technique,
manipulation has
no contraindications and can be applied to all tissues of the body.
Pre-treatment assessment
should be performed as part of each manipulative treatment visit to ensure that
the correct
diagnosis and correct treatment is employed.
High
velocity, low amplitude (HVLA) manipulation is performed by taking a joint to
its end range of
motion and moving the articulation into the zone of accessory joint movement,
well within the limits
of anatomical integrity. Indications for manipulation include joint pain,
decreased joint motion, and
joint adhesions. Contraindications to HVLA manipulation include joint
instability, fractures, severe
osteoporosis, infection, metastatic cancer, active inflammatory arthritides,
aortic aneurysm, and
signs of progressive neurologic deficits.
Time
to produce effect for all types of manipulative treatment: 1 to 6 treatments.
Frequency: Up to 3 times per week for
the first 4 weeks as indicated by the severity of
involvement
and the desired effect, then up to 2 treatments per week for the next 4 weeks.
For
further treatments, twice per week or less to maintain function.
Maximum duration: 36 visits. Extended durations of care beyond what is
considered “maximum”
may
be necessary in cases of re-injury, interrupted continuity of care,
exacerbation of symptoms,
and in those patients with comorbidities. Refer to the Chronic Pain Guidelines
for care beyond 6
months.
The
combination of 97140 plus either CMT or OMT code is equal to one visit when
performed on the same day. Any combination of manual therapeutic intervention
exceeding 36 visits (not units) need to go to UR.
5.10.3.1 Mobilization
(Joint) /Manipulation Mobilization is passive movement involving oscillatory motions
to the involved joints. The passive mobility is performed in a graded manner
(I, II, III, IV, or V), which depicts the speed of the maneuver. It may include
skilled manual joint tissue stretching. Indications
include the need to improve joint play, improve intracapsular
arthrokinematics, or reduce pain associated with tissue impingement. Time to
produce effect: 4 to 6 treatments Frequency: 2 to 3 times per week Maximum
duration: 36 visits (CPT codes 97124 and 97140 can not exceed 36 visits in combination).
5.10.4 Massage — Manual or
Mechanical: Massage is manipulation of soft tissue with broad ranging
relaxation and circulatory benefits. This may include techniques that include
pressing, lifting, rubbing, pinching of soft tissues by, or with, the
practitioner's hands. Indications include edema (peripheral or hard and
non-pliable edema), muscle spasm, adhesions, the need to improve peripheral
circulation and range of motion, or to increase muscle relaxation and flexibility
prior to exercise.
In
sub-acute low back pain populations there is good evidence that massage can
increase function when combined with exercise and patient education. Some
studies have demonstrated a decrease in provider visits and pain medication use
with combined therapy. One study indicated improved results with acupressure
massage. It is recommended that all massage be performed by trained,
experienced therapists and be accompanied by an active exercise program and
patient education. In contrast to the sub-acute population, massage is a
generally accepted treatment for the acute low back pain population, although
no studies have demonstrated its efficacy for this set of patients.
Time
to produce effect: Immediate Frequency: 1 to 3 times per week Maximum duration:
12 visits (CPT codes 97124 and 97140 can not exceed 36 visits in
combination).
5.10.5 Mobilization (Joint): is
a generally well-accepted treatment. Mobilization is passive movement involving
oscillatory motions to the vertebral segment(s). The passive mobility is
performed in a graded manner (I, II, III, IV, or V), which depicts the speed
and depth of joint motion during the maneuver. For further discussion on Level
V joint mobilization please see section on HVLA manipulation [Refer to section
12. d.]. It may include skilled manual joint tissue stretching. Indications
include the need to improve joint play, segmental alignment, improve
intracapsular arthrokinematics, or reduce pain associated with tissue impingement.
Mobilization should be accompanied by active therapy. For Level V mobilization
contraindications include joint instability, fractures, severe osteoporosis,
infection, metastatic cancer, active inflammatory arthritides, aortic aneurysm,
and signs of progressive neurologic deficits. Time to produce effect for all
types of manipulative treatment: 1 to 6 treatments. Frequency: Up to 3 times
per week for the first 4 weeks as indicated by the severity of involvement and
the desired effect, then up to 2 treatments per week for the next 4 weeks. For
further treatments, twice per week or less to maintain function. Maximum
duration: 36 visits. Extended durations of care beyond what is considered
“maximum” may be necessary in cases of re-injury, interrupted continuity of
care, exacerbation of symptoms, and in those patients with comorbitities. Refer
to the Chronic Pain Guidelines for care beyond 6 months.
RE-EVALUATE TREATMENT EVERY 3 TO 4 WEEKS If a given treatment or modality is not
producing positive results within 3 to 4 weeks, the treatment may be either
modified or discontinued. Reconsideration of diagnosis should also occur in the
event of poor response to a seemingly rational intervention.
CPT
codes 97124 and 97140 can not exceed 36 visits in combination
5.10.6 Mobilization (Soft
Tissue): is a generally well-accepted treatment. Mobilization of soft
tissue is the skilled application of muscle energy, strain/counter strain,
myofascial release, manual trigger point release, and manual therapy techniques
designed to improve or normalize movement patterns through the reduction of
soft tissue pain and restrictions. These can be interactive with the patient
participating or can be with the patient relaxing and letting the practitioner
move the body tissues. Indications include muscle spasm around a joint, trigger
points, adhesions, and neural compression. Mobilization should be accompanied
by active therapy.
Maximum duration: 36 visits RE-EVALUATE
TREATMENT EVERY 3 TO 4 WEEKS If a given treatment or modality is not
producing positive results within 3 to 4 weeks, the treatment may be either
modified or
discontinued.
Reconsideration of diagnosis should also occur in the event of poor response to
a
seemingly rational intervention.
CPT codes 97124 and 97140 can not exceed 36 visits in combination.
5.10.7 Short-Wave
Diathermy: is an accepted treatment which involves the use of equipment
that exposes soft tissue to a magnetic or electrical field. Indications include
enhanced collagen extensibility before stretching, reduced muscle guarding,
reduced inflammatory response, and enhanced re-absorption of
hemorrhage/hematoma or edema. It is an accepted modality as an adjunct to
acupuncture or situation where other forms of contact superficial heat are
contraindicated.
5.10.8 Superficial
Heat and Cold Therapy (excluding Infrared Therapy): is a generally accepted
treatment. Superficial heat and cold are thermal agents applied in various
manners that lower or raise the body tissue temperature for the reduction of
pain, inflammation, and/or effusion resulting from injury or induced by
exercise. Includes application of heat just above the surface of the skin at
acupuncture points. Indications include acute pain, edema and hemorrhage, need
to increase pain threshold, reduce muscle spasm, and promote
stretching/flexibility. Cold and heat packs can be used at home as an extension
of therapy in the clinic setting.
Time
to produce effect:
Immediate
Frequency: 2 to 5 times per week
Maximum duration: 12 visits with a maximum of 1 unit per day.
5.10.9 Traction—Manual:
is an accepted treatment and an integral part of manual manipulation or joint
mobilization. Indications include decreased joint space, muscle spasm around
joints, and the need for increased synovial nutrition and response. Manual
traction is contraindicated in patients with tumor, infection, fracture, or
fracture dislocation.
5.10.10 Traction—Mechanical:
Traction modalities are contraindicated in patients with tumor, infections,
fracture, or fracture dislocation. Non-oscillating inversion traction methods
are contraindicated in patients with glaucoma or hypertension. Motorized
traction/decompression devices are included (i.e. VAX-D, DRX9000,etc.) A home
lumbar traction unit can be purchased if therapy proves effective.
Time to produce effect: 1 to 3 sessions up
to 30 minutes. If response is negative after 3 treatments, discontinue this
modality. Frequency: 2 to 3 times per week. A home lumbar traction unit can be
purchased if therapy proves effective. Maximum duration: 24 visits
5.10.11 Transcutaneous
Electrical Nerve Stimulation (TENS): is a generally accepted treatment.
TENS should include at least one instructional session for proper application
and use. Indications include muscle spasm, atrophy, and decreased circulation and
pain control. Minimal TENS unit parameters should include pulse rate, pulse
width and amplitude modulation. Consistent, measurable functional improvement
should be documented prior to the purchase of a home unit.
Time to produce effect: Immediate
Frequency: Variable
5.10.12 Ultrasound
(Including Phonophoresis): is an accepted treatment. Ultrasound uses sonic
generators to deliver acoustic energy for therapeutic thermal and/or
non-thermal soft tissue effects. Indications include scar tissue, adhesions,
collagen fiber and muscle spasm, and the need to extend muscle tissue or
accelerate the soft tissue healing. Ultrasound with electrical stimulation is
concurrent delivery of electrical energy that involves dispersive electrode
placement. Indications include muscle spasm, scar tissue, pain modulation, and
muscle facilitation.
Phonophoresis is the transfer of medication
to the target tissue to control inflammation and pain through the use of sonic
generators. These topical medications include, but are not limited to,
steroidal
anti-inflammatory and anesthetics. Phonopheresis is not recommended for Low
Back Pain. Time to produce effect: 6 to 15 treatments Frequency: 3 times per
week Maximum duration: 18 visits
5.10.13 Whirlpool/Hubbard
Tank: is a generally accepted treatment in which conductive exposure to
water at varied temperatures that best elicits the desired effect. It generally
includes massage by water propelled by a turbine or Jacuzzi jet system and has
the same thermal effects as hot packs, if water temperature exceeds tissue
temperature. It has the same thermal effects as cold application, if comparable
temperature water is used. Indications include the need for analgesia, relaxing
muscle spasm, reducing joint stiffness, and facilitating and preparing for
exercise. This is not recommended for Low Back Pain.
5.11 THERAPY—ACTIVE
The following active therapies are widely used and accepted methods of care for
a variety of work-related injuries. They are based on the philosophy that
therapeutic exercise and/or activity are beneficial for restoring flexibility,
strength, endurance, function, range of motion, and can alleviate discomfort.
Active therapy requires an internal effort by the individual to complete a specific
exercise or task. This form of therapy requires supervision from a therapist or
medical provider such as verbal, visual, and/or tactile instruction(s). At
times, the provider may help stabilize the patient or guide the movement
pattern but the energy required to complete the task is predominately executed
by the patient.
Patients should be instructed to continue
active therapies at home as an extension of the treatment process in order to
maintain improvement levels. Follow-up visits to reinforce and monitor progress
and proper technique are recommended. Home exercise can include exercise with
or without mechanical assistance or resistance and functional activities with
assistive devices. The following active therapies are listed in alphabetical
order:
5.11.1 Activities
of Daily Living (ADL) are well-established interventions which involve
instruction, active-assisted training, and/or adaptation of activities or
equipment to improve a person's capacity in normal daily activities such as
self-care, work re-integration training, homemaking, and driving.
Time to produce effect: 4 to 5 treatments
Maximum duration: 10 visits
5.11.2 Aquatic
Therapy: is a well-accepted treatment which consists of the therapeutic use
of aquatic immersion for therapeutic exercise to promote strengthening, core
stabilization, endurance, range of motion, flexibility, body mechanics, and
pain management. Aquatic therapy includes the implementation of active
therapeutic procedures in a swimming or therapeutic pool. The water provides a
buoyancy force that lessens the amount of force gravity applies to the body.
The decreased gravity effect allows the patient to have a mechanical advantage
and more likely have a successful trial of therapeutic exercise. The therapy
may be indicated for individuals who:
Cannot tolerate active land-based or
full-weight bearing therapeutic procedures Require increased support in the
presence of proprioceptive deficit; Are at risk of compression fracture due to
decreased bone density; Have symptoms that are exacerbated in a dry
environment; Would have a higher probability of meeting active therapeutic
goals than in a land-based environment.
The pool should be large enough to allow
full extremity range of motion and fully erect posture. Aquatic vests, belts
and other devices can be used to provide stability, balance, buoyancy, and
resistance.
Time
to produce effect: 4 to 5 treatments Frequency: 3 to 5 times per week Maximum
duration: 20 visits A self-directed program is recommended after the supervised
aquatics program has been
established, or, alternatively a transition
to a land-based environment exercise program.
5.11.3 Functional
Activities: are well-established interventions which involve the use of
therapeutic activity to enhance mobility, body mechanics, employability,
coordination, balance, and sensory motor integration.
Time
to produce effect: 4 to 5 treatments Frequency: 3 to 5 times per week Maximum
duration: 24 visits Total number of visit 97110 and 97530 should not exceed 36
visits without pre-authorization.
5.11.4 Functional
Electrical Stimulation: is an accepted treatment in which the application
of electrical current to elicit involuntary or assisted contractions of
atrophied and/or impaired muscles. It may be indicated for impaired muscle
function to radiculopathy. (Foot drop)
Time
to produce effect: 2 to 6 treatments Frequency: 3 times per week Maximum
duration: 14 visits inclusive of electrical muscle stimulation codes if
beneficial provide
with
home unit.
5.11.5 Neuromuscular
Re-education: is a generally accepted treatment. It is the skilled
application of exercise with manual, mechanical, or electrical facilitation to
enhance strength; movement patterns; neuromuscular response; proprioception,
kinesthetic sense, coordination; education of movement, balance, and posture.
Indications include the need to promote neuromuscular responses through
carefully timed proprioceptive stimuli, to elicit and improve motor activity in
patterns similar to normal neurologically developed sequences, and improve
neuromotor response with independent control.
Time
to produce effect: 2 to 6 treatments
Frequency: 3-5 times per week
Maximum duration: 30 visits
5.11.6 Therapeutic
Exercise: is a generally well-accepted treatment. Therapeutic exercise,
with or without mechanical assistance or resistance, may include isoinertial,
isotonic, isometric and isokinetic types of exercises. Indications include the
need for cardiovascular fitness, reduced edema, improved muscle strength, improved
connective tissue strength and integrity, increased bone density, promotion of
circulation to enhance soft tissue healing, improvement of muscle recruitment,
improved proprioception, and coordination, increased range of motion.
Therapeutic exercises are used to promote normal movement patterns, and can
also include complementary/ alternative exercise movement therapy (with
oversight of a physician or appropriate healthcare professional).
Spinal
Stabilization: is a generally well-accepted treatment. The goal of this
therapeutic program is to strengthen the spine in its neural and anatomic
position. The stabilization is dynamic which allows whole body movements while
maintaining a stabilized spine. It is the ability to move and function normally
through postures and activities without creating undue vertebral stress
Time
to produce effect: 2 to 6 treatments Frequency: 3 to 5 times per week Maximum
duration: 30 visits Total number of visits of 97110 & 97530 may not exceed
36 visits without pre-authorization.
5.12 VOCATIONAL
REHABILITATION is a generally accepted intervention. Initiation of
vocational rehabilitation requires adequate evaluation of patients for
quantification of highest functional level, motivation, and achievement of
maximum medical improvement. Vocational rehabilitation may be as simple as
returning to the original job or as complicated as being retrained for a new
occupation.
It
may also be beneficial for full vocational rehabilitation to be started, if it is evident that the injured worker will be unable to return to
his/her previous occupation. A positive goal and direction may aid the patient
in decreasing stress and depression, and promote optimum rehabilitation.
All
operative interventions must be based upon positive correlation of clinical
findings, clinical course, and diagnostic tests. A comprehensive assimilation
of these factors must lead to a specific diagnosis with positive identification
of pathologic condition(s). It is important to consider non-physiologic
modifiers of pain presentation or non-operative conditions mimicking
radiculopathy or instability prior to consideration of elective surgical
intervention.
While
sufficient time allowances for non-operative treatment are required to
determine the natural cause and response to non-operative treatment of low back
pain disorders, timely decision making for operative intervention is critical
to avoid de-conditioning and increased disability (exclusive of
"emergent" or urgent pathology such as cauda equina syndrome or
associated rapidly progressive neurologic loss).
In
general, if the program of non-operative treatment fails, operative treatment
is indicated when: Improvement of the symptoms has plateaued and the residual
symptoms of pain and functional disability are unacceptable at the end of
active treatment, or at the end of longer duration of non-operative programs
for debilitated patients with complex problems; and/or Frequent recurrences of
symptoms cause serious functional limitations even if a non-operative active
treatment program
provides
satisfactory relief of symptoms, and restoration of function on each
recurrence. Mere passage of time with poorly guided treatment is not considered
an active treatment program. Surgical evaluation for simple decompression of
patients with herniated nucleus pulposus and sciatica
should
occur within 6 to 12 weeks after injury at the latest, within the above stated
contingencies. For patients with true, refractory mechanical low back pain in
whom fusion is being considered, it is recommended that a surgical evaluation
or interventions occur within 4 months following injury.
Spinal
decompression surgeries and fusion have re-operation rates of approximately 10%
or more over the following five years. Re-operation is indicated only when the
outcome following the re-operation is expected to be better, within a
reasonable degree of certainty, than the outcome of other non-invasive or less
invasive treatment procedures. “Outcomes” refer to the patient’s ability to
improve functional tolerances such as sitting, standing, walking, strength,
endurance, and/or vocational status and pain level. While timely surgical
decision-making is critical to avoid de-conditioning and increased disability,
a time limited trial of reconditioning may be tried prior to re-operation.
Every
post-operative patient should be involved in an active treatment program. The
non-surgical rehab guidelines listed above do not apply to post-operative
rehabilitation and work conditioning. Interdisciplinary interventions should be
strongly considered post-operatively in any patient not making functional
progress within expected time frames.
6.1 DISCECTOMY
AND NERVE ROOT DECOMPRESSION
6.1.1 Description:
To enter into and partially remove the disc and/or Decompress Nerve Root.
6.1.2 Surgical
Indications: May include any of the following: Primary radicular symptoms,
radiculopathy on exam, correlating imaging study, and failure of non-surgical
care.
6.1.3 Post-Operative
Therapy: A formal physical therapy
program should be implemented postoperatively. Active treatment, which
patients should have had prior to surgery, will frequently require a repeat of
the visits previously ordered. The non-surgical rehab guidelines listed above
do not apply to post-operative rehabilitation and work conditioning
6.2 PERCUTANEOUS
DISCECTOMY
6.2.1 Description:
An invasive operative procedure to accomplish partial removal of the disc
through a needle which allows aspiration of a portion of the disc trocar under
imaging control.
6.2.2 Surgical
Indications: Percutaneous discectomy is indicated only in cases of
suspected septic discitis in order to obtain diagnostic tissue. The procedure
is not recommended for contained disc herniations or bulges with associated
radiculopathy due to lack of evidence to support long-term improvement.
6.3 LAMINOTOMY/LAMINECTOMY/FORAMENOTOMY/FACETECTOMY
6.3.1 Description:
These procedures provide access to produce neural decompression by partial
or total removal of various parts of vertebral bone.
6.3.2 Surgical
Indications: May include all of the following: Primary radicular symptoms,
radiculopathy on exam, correlating imaging study, and failure of non-surgical
care.
6.3.3 Post-Operative
Therapy: A formal rehab program should be implemented post-operatively.
Active treatment, which patients should have had prior to surgery, will
frequently require a repeat of the visits previously ordered. The
implementation of a gentle aerobic reconditioning program (e.g., walking) and
back education within the first post-operative week is appropriate in
uncomplicated post-surgical cases. Some patients may benefit from several
occupational therapy visits to improve performance of ADLs. Participation in an
active therapy program which includes restoration of ROM, core stabilization,
strengthening, and endurance is recommended. The goals of the therapy program
should include instruction in a long-term home based exercise program. The
non-surgical physical therapy guidelines listed above do not apply to
post-operative rehabilitation and work conditioning
6.4 SPINAL
FUSION
6.4.1 Description:
Production of a rigid connection between two or more adjacent vertebrae.
6.4.2 Surgical
Indications: A timely decision-making process is recommended when
considering patients for possible fusion. For chronic low back problems, fusion
should not be considered within the first 4 months of symptoms, except for
fracture, dislocation, recurrent herniation, or gross instability
Indications for spinal fusion may include:
6.4.2.1 Neural
arch defect – Spondylolytic spondylolisthesis, congenital unilateral neural
arch hypoplasia.
6.4.2.2 Segmental
Instability - Excessive motion, as in degenerative spondylolisthesis,
surgically induced segmental instability.
6.4.2.3 Primary
Mechanical Back Pain/Functional Spinal Unit Failure - Multiple pain generators
objectively involving two or more of the following: (a) internal disc
disruption (poor success rate if more than two disc involved), (b) painful
motion segment, as in annular tears, (c) disc resorption, (d) facet syndrome,
and or (e) ligamentous tear. (f) Degenerative disc disease.
6.4.2.4 Revision
surgery for failed previous operation(s) if significant functional gains are
anticipated.
6.4.2.5 History
of multiple recurrent herniated discs.
6.4.3 Pre-operative
Surgical Indications: Required pre-operative clinical surgical indications
for spinal fusion include all of the following:
6.4.3.1 Planned
fusion to exceed two levels requires confirmatory second opinion.
6.4.3.2 For
any potential fusion surgery, it is recommended that the injured worker be
encouraged to refrain from smoking for at least six weeks prior to surgery and
during the period of fusion healing. Because smokers have a higher risk of
non-union and higher postoperative costs, it is recommended that insurers
cover a smoking cessation program perioperatively.
6.4.4 Post-operative
Therapy: A formal rehab program should be implemented post-operatively.
Active treatment, which patients should have had prior to surgery, will
frequently require a repeat of the visits previously ordered. The
implementation of a gentle aerobic reconditioning program (e.g., walking), and
back education within the first post-operative week is appropriate in
uncomplicated post-surgical cases. Some patients may benefit from several
occupational therapy visits to improve performance of ADLs. Participation in an
active therapy program which includes core stabilization, strengthening, and
endurance is recommended the goals of the therapy program should include
instruction in a long-term home-based exercise program. The non-surgical
physical therapy guidelines listed above do not apply to post-operative
rehabilitation and work conditioning
6.4.5 Return-to-Work:
Barring complications, patients responding favorably to spinal fusion may be
able to return to sedentary-to-light work within 6 to 12 weeks
post-operatively, light-to-medium work within 6 to 9 months post-operatively
and medium-to-medium/heavy work within 6 to 12 months post-operatively.
Patients requiring fusion whose previous occupation involved heavy-tovery-heavy
labor should be considered for vocational assessment as soon as reasonable
restrictions can be predicted. The practitioner should release the patient with
specific physical restrictions and should obtain a clear job description from
the employer, if necessary. Once an injured worker is off work greater than 6
months, the functional prognosis with or without fusion becomes guarded for
that individual.
6.5 SACROILIAC
JOINT FUSION
6.5.1 Description:
Use of bone grafts, sometimes combined with metal devices, to produce a
rigid connection between two or more adjacent vertebrae providing symptomatic
instability as a part of major pelvic ring disruption.
6.5.2 Surgical
Indications: Sacroiliac (SI) joint fusion may be indicated for
stabilization of a traumatic severe disruption of the pelvic ring. This
procedure has limited use in minor trauma and would be considered only on an
individual case-by-case basis. In patients with typical mechanical low back
pain, this procedure is considered to be investigational. Until the efficacy of
this procedure for mechanical low back pain is determined by an independent
valid prospective outcome study, this procedure is not recommended for
mechanical low back pain.
6.6 IMPLANTABLE
SPINAL CORD STIMULATORS are reserved for those low back pain patients with
pain of greater than 6 months duration who have not responded to the standard non-operative
or operative interventions previously discussed within this document. Refer to
Division’s Chronic Pain Disorder Medical Treatment Guidelines.
6.7 INTRADISCAL
ELECTROTHERMAL ANNULOPLASTY (IDEA) (more commonly called IDET, or
Intradiscal Electrothermal therapy)
6.7.1 Description:
An outpatient non-operative procedure. A wire is guided into the identified
painful disc using fluoroscopy. The wire is then heated at the nuclear annular
junction within the disc. Physicians performing this procedure must have been
trained in the procedure and should have performed at least 25 prior
discograms. Prior authorization is required for IDET.
6.7.2 Surgical
Indications: Failure of conservative therapy including physical therapy,
medication management, or therapeutic injections. Indications may include those
with chronic low back pain, disc related back pain, or pain lasting greater
than 6 months. There is conflicting evidence regarding its effectiveness. In
one of the most recent studies only approximately 40% of patients had greater
than 50% relief of pain. Patients should be aware of these percentages. Strict
adherence to the indications is recommended
The candidate should meet the following
criteria:
6.7.2.1 Age
not above 60 or under 18; and
6.7.2.2 Normal
neurological exam; and
6.7.2.3 No
evidence of nerve root compression on MRI; and
6.7.2.4 Concordant
pain reproduced with provocation discography (low pressure); and
6.7.2.5 Functionally
limiting low back pain far in excess of leg pain for at least 6 months; and
6.7.2.6 No
evidence of inflammatory arthritis, spinal conditions mimicking low back pain,
moderate to severe spinal stenosis, spinal instability, disc herniation, or
medical or metabolic diseases precluding follow-up rehabilitation; and
6.7.2.7 Disc
height greater than 50% of adjacent normal disc; and
6.7.2.8 No
previous IDET procedure at the same level.
6.7.3 Post-Procedure
Therapy: Some cases may require epidural injection after the IDET procedure
has been performed. A corset should be used for the first 6 weeks. Sitting
upright is limited to 30 to 45 minutes for the first two weeks. A formal
physical therapy program should be implemented post-operatively. Some patients
may benefit from several occupational therapy visits to improve performance of
ADLs. Rehabilitation may take as long as 6 months and include stretching during
the first month, floor exercises in the second month, 3 to 5 consecutive months
of progressive exercise program, and sport activities in the 5th and 6th months
as tolerated. The goals of the therapy program should include instruction in a
long-term home-based exercise program. The non-surgical physical therapy
guidelines listed above do not apply to post-operative rehabilitation and work
conditioning
Return to Work: Barring complications, may be able to return
to limited duty after one to two weeks. A corset should be used for the first
six weeks. Sitting upright is limited to 30 to 45 minutes for the first two weeks.
Zero to 10 pounds lifting limits for first 6 weeks post-procedure. If
successful, patients may return to medium work category (20 to 50 pounds per
DOT standards) at 4 to 6 months.
6.8 LASER
DISCECTOMY involves the delivery of laser energy into the center of the
nucleus pulposus using a fluoroscopically guided laser fiber under local
anesthesia. The energy denatures protein in the nucleus, causing a structural
change which is intended to reduce intradiscal pressure. Its effectiveness has
not been shown. Laser discectomy is not recommended.
6.9 ARTIFICIAL
LUMBAR DISC REPLACEMENT
6.9.1 Description:
involves the insertion of a prosthetic device into an
intervertebral space from which a degenerated disc has been removed, sparing only the
peripheral annulus. The endplates are positioned under intraoperative fluoroscopic
guidance for optimal placement in the sagittal and frontal planes. The prosthetic device is
designed to distribute the mechanical load of the vertebrae in a physiologic manner and
maintain range of motion.
General selection criteria for lumbar disc replacement includes symptomatic
one-level degenerative disc disease. The patient must also meet fusion surgery criteria, and if the
patient is not a candidate for fusion, a disc replacement procedure should not be
considered. Additionally, the patient should be able to comply with pre-and post-surgery
protocol.
The theoretical advantage of total disc arthroplasty is that it preserves range of
motion and physiologic loading of the disc. This could be an advantage for adults who are
physically active. Studies do not demonstrate a long-term advantage of measured
function or pain over comparison groups undergoing fusion. The longevity of this
prosthetic device has not yet been determined. Significant technical training and
experience is required to perform this procedure successfully. Surgeons must be well-
versed in anterior spinal techniques and should have attended appropriate training
courses, or have undergone training during a fellowship. Mentoring and proctoring of
procedures is highly recommended. Reasonable pre-operative evaluation may include an
angiogram to identify great vessel location. The angiogram may be either with contrast or with magnetic resonance imaging. An assistant surgeon with anterior access experience
is required.
6.9.2 Surgical
Indications:
Symptomatic one-level
degenerative disc disease established by objective testing (CT or MRI
scan followed by positive provocation discogram, if necessary). Symptoms unrelieved after six
months of active non-surgical treatment Physical medicine and manual therapy interventions.
6.9.3 Contraindications:
Significant spinal deformity/scoliosis Facet joint arthrosis Spinal
instability Deficient posterior elements Infection Any contraindications to an
anterior abdominal approach (including multiple prior abdominal procedures)
Previous compression or burst fracture at the surgical level Spinal canal
stenosis Spondylolysis Spondylolisthesis greater than 3 mm Osteoporosis or any
metabolic bone disease Chronic steroid use or use of other medication known to
interfere with bone or soft
tissue healing Autoimmune disorder Allergy
to device components/materials Morbid obesity (e.g., body/mass index [BMI] of
greater than 40, over 100 pounds overweight) Active malignancy
6.9.4 Post-operative
Therapy: Bracing may be appropriate. A formal therapy program
should be implemented post-operatively. Active treatment, which patients may
have had prior to surgery, will frequently require a repeat of the visits
previously ordered. The implementation of a gentle aerobic reconditioning
program (e.g., walking) and back education within the first postoperative week
is appropriate in uncomplicated post-surgical cases. Some patients may benefit
from several occupational therapy visits to improve performance of ADLs.
Participation in an active therapy program which includes restoration of ROM,
core stabilization, strengthening, and endurance is recommended to be initiated
at the discretion of the surgeon. Lifting and bending are usually limited for
several months at least. Sedentary duty may be able to begin within six weeks
in uncomplicated cases. The goals of the therapy program should include
instruction in a long-term home based exercise program. The non-surgical
therapy guidelines listed above do not apply to post-operative
rehabilitation and work conditioning
6.10 KYPHOPLASTY
6.10.1 Description:
A surgical procedure for the treatment of symptomatic thoracic or lumbar
vertebral compression fractures, most commonly due to osteoporosis or other
metabolic bone disease, and occasionally with post-traumatic compression
fractures and minor burst fractures that do not significantly compromise the
posterior cortex of the vertebral body. Pain relief can be expected in approximately
90% of patients. Vertebral height correction is inconsistent, with
approximately 35% to 40% of procedures failing to restore height or kyphotic
angle.
6.10.2 Operative
Treatment: Kyphoplasty involves the percutaneous insertion of a trocar and inflatable
balloon or expanding polymer into the vertebral body, which re-expands the
body, elevating the endplates and reducing the compression deformity.
Polymethylmethacrylate (PMMA) bone cement is injected under low pressure into
the cavity created by the balloon inflation. In contrast to vertebroplasty,
which introduces PMMA cement under high pressure, the space created by balloon
inflation allows a higher viscosity PMMA to be injected under lower pressure,
which may reduce the risks associated with extravertebral extravasation of the
material. There may be an advantage to performing the procedure within one
month of the fracture, since the elevation of the endplates may be more readily
achieved than when the procedure is delayed.
6.10.3 Surgical
Indications: Kyphoplasty is an accepted treatment for the following
indications: Compression fracture vertebral height loss between 20% and 85%
Vertebral height restoration. Kyphoplasty is more likely to increase vertebral
height if performed within 30 days of fracture
occurrence
6.10.4 Contraindications:
The presence of neurologic compromise related to fracture
High-velocity fractures with a significant burst component
Significant posterior vertebral body wall fracture
Severe vertebral collapse (vertebra plana)
Infection, and
Coagulopathy
6.11 VERTEBROPLASTY
6.11.1 Description:
a procedure for the treatment of painful thoracic and lumbar vertebral
compression fractures caused by osteoporosis or other metabolic bone disease.
Polymethylmethacrylate (PMMA) bone cement is injected with high pressure into
the vertebral body via an 11- to 13-gauge needle, with the goal of stabilizing
the spine and relieving pain. The procedure does not correct spinal deformity.
Pain relief can be expected in approximately 90% of patients. Vertebral height
correction is inconsistent, with approximately 35% to 40% of procedures failing
to restore height or kyphotic angle.
6.11.2 Indications:
Compression
fracture of preferably less than 30 days Vertebral height loss between 20% and
85% Intact posterior wall
6.11.3 Contraindications:
The presence of neurologic compromise related to the fracture; High
velocity fractures with a
significant
burst component. Posterior vertebral body wall fracture; Severe vertebral
collapse (vertebra plana); and Infection; and Coagulopathy
6.12 PERCUTANEOUS
RADIOFREQUENCY DISC DECOMPRESSION is an investigational procedure which
introduces a 17 gauge cannula under local anesthesia and fluoroscopic guidance
into the nucleus pulposus of the contained herniated disc, using radiofrequency
energy to dissolve and remove disc material. Pressure inside the disc is
lowered as a result. There have been no randomized clinical trials of this
procedure at this time. Percutaneous radiofrequency disc decompression is not
recommended.
6.13 NUCLEUS
PULPOSUS REPLACEMENT involves the introduction of a prosthetic implant into
the intervertebral disc, replacing the nucleus while preserving the annulus
fibrosus. It is limited to investigational use in the United States
at this time. It is not recommended.
6.14 EPIDUROSCOPY
AND EPIDURAL LYSIS OF ADHESIONS (Refer to Injections-Therapeutic).
6.15 INTRAOPERATIVE
MONITORING is a common intraoperative electrodiagnostic technique that may
include somatosensory evoked potentials (SSEP), motor evoked potentials (MEP),
or pedicle screw monitoring. The monitoring procedure may be used to evaluate
spinal cord integrity and screw placement during the operative procedure. The
use of intraoperative monitoring can be anticipated to become more common as
percutaneous spinal procedures gain greater acceptance.
7.1 Global
Reimbursement
The
reimbursement allowances for surgical procedures are based on a global
reimbursement concept that covers performing the basic service and the normal
range of care required after surgery. Global reimbursement includes:
7.1.1 The
operation per se
7.1.2 Local
infiltration, metacarpal/metatarsal/digital block or topical anesthesia
7.1.3 Subsequent
to the decision and/or authorization for surgery, one related E/M encounter on
the date immediately prior to or on the date of the procedure (including
history and physical), but does not include the initial consultation
7.1.4 Immediate
postoperative care, including dictating operative notes, talking with the
family and other physicians
7.1.5 Writing
orders
7.1.6 Evaluating
the patient in the post anesthesia recovery area
7.1.7 Normal, uncomplicated
follow-up care for the time periods indicated in the follow- up days (FUD)
column to the right of each procedure code. The number in that column
establishes the days during which no additional reimbursement is allowed for
the usual care provided following surgery, absent complications or unusual
circumstances.
7.1.8 The
maximum reimbursement allowances cover all normal postoperative care, including
the
removal
of sutures by the surgeon or associate. Follow-up days are specified by
procedure. Follow-up days listed are for 0, 10, or 90 days and are listed in
the Fee Schedule as 000, 010, or
090.
7.2 Implants
Bone morphogenetic protein is an FDA approved biologic fusion and fracture
healing aid. Its use in spine and fracture surgery represents the standard of
care in our community, and in both on-label and off-label applications is
accepted and to be reimbursed to the facility providing the implant, at rates
consistent with implant payment rates
determined under the respective ASC and hospital reimbursement guidelines
7.3 Surgical
Assistant
7.3.1 Physician
surgical assistant — For the purpose of reimbursement, a physician who assists
at surgery is reimbursed as a surgical assistant. Assistant surgeons should use
modifier 80 and are allowed twenty percent (20%) of the maximum reimbursement
allowance (MRA) for the procedure(s).
7.3.2 Registered
Nurse Surgical Assistant or Physician Assistant
7.3.2.1 A
physician assistant, or registered nurses who have completed an approved first
assistant training course, may be allowed a fee when assisting a surgeon in the
operating room (O.R.).
7.3.2.2 The
maximum reimbursement allowance for the physician assistant or the registered
nurse first assistant (RNFA) is twenty percent (20%) of the surgeon’s fee for
the procedure(s) performed.
7.3.2.3 Under
no circumstances will a fee be allowed for an assistant surgeon and a physician
assistant or RNFA at the same surgical encounter.
7.3.2.4 Registered
nurses on staff in the O.R. of a hospital, clinic, or outpatient surgery center
do not qualify for reimbursement as an RNFA.
7.4 Therapeutic Procedures
Therapeutic procedures (injecting into cavities, nerve blocks, etc.) (CPT
codes 20526–20610, 64400, 64450) may be billed in addition to the medical care
for a new patient. (Use appropriate level of service plus injection.) In
follow-up cases for additional therapeutic injections and/or aspirations, an
office visit is only indicated if it is necessary to re-evaluate the patient.
In this case, a minimal visit may be listed in addition to the injection.
Documentation supporting the office visit charge must be submitted with the
bill to the payer. This is clarified in the treatment guidelines in a more
specific manner. Trigger point injection is considered one procedure and
reimbursed as such regardless of the number of injection
sites.
Two codes are available for reporting trigger point injections. Use 20552 for
injection(s) of single or multiple trigger point(s) in one or two muscles or
20553 when three or more muscles are involved.
7.5 Intervertebral
Biomechanical Device(s) and Use of Code 22851 Code 22851 describes the
application of an intervertebral biomechanical device to a vertebral defect or
interspace. Code 22851 should be listed in conjunction with a primary procedure
without the use of modifier 51. The use of 22851 is limited to one instance per
single interspace or single vertebral defect regardless of the number of
devices applied and infers additional qualifying training, experience, sizing,
and/or use of special surgical appliances to insert the biomechanical device.
Qualifying devices include manufactured synthetic or allograft biomechanical
devices, or methyl methacrylate constructs, and are not dependant on a specific
manufacturer, shape, or material of which it is constructed. Qualifying devices
are machine cut to specific dimensions for precise application to an
intervertebral defect. (For example, the use of code 22851 would be appropriate
during a cervical arthrodesis (22554) when applying a synthetic alloy cage, a
threaded bone dowel, or a machine cut hexahedron cortical, cancellous, or
cortico cancellous allograft biomechanical device. Surgeons utilizing generic
non-machined
bony allografts or autografts are referred to code sets 20930–20931, 20936–
20938respectively.)
7.6 Spinal and Cranial
Services Require Additional Surgeon Certain spinal and cranial procedures
require the services of an additional surgeon of a different specialty to gain
exposure to the spine and brain. These typically are vascular, thoracic and
ENT. The
surgical
exposure portion of these procedures will be billed, dictated and followed
separately by the exposure surgeon for their portion of the procedure.
7.7 Multiple Procedure
Reimbursement Rule
Multiple procedures performed during the
same operative session at the same operative site are reimbursed at 100% of the
allowable fee for the primary and all subsequent procedures.
7.8 External Spinal
Stimulators Post Fusion
7.8.1 The
following criteria are established for the medically accepted standard of care
when determining applicability for the use of an external spinal stimulator.
However, the medical necessity should be determined on a case-by-case basis.
7.8.1.1 Patient
has had a previously failed spinal fusion, and/or
7.8.1.2 Patient
is scheduled for revision or repair of pseudoarthrosis, and/or
7.8.1.3 The
patient smokes greater than a pack of cigarettes per day and is scheduled for
spinal fusion
7.8.2 The
external spinal stimulator is approved for use in primary spinal fusions, if
medical co morbidities increase the likelihood of non-union
7.8.3 The
external spinal stimulator will be reimbursed by report (BR).
7.8.4 The
patient is metabolically in poor health, with other medical co morbidities such
as diabetes, Rheumatoid arthritis, lupus or other illnesses requiring oral
steroids or cytotoxic medications.
7.8.5 Precertification
is required for use of the external spinal stimulator if the planned use falls
outside the above indications.
PART E SHOULDER TREATMENT GUIDELINES
Pursuant to 19 Del.C. §2322C, health
care practice guidelines have been adopted and recommended by the Health Care
Advisory Panel to guide utilization of health care treatments in workers'
compensation including, but not limited to, care provided for the treatment of
employees by or under the supervision of a licensed health care provider,
prescription drug utilization, inpatient hospitalization and length of stay,
diagnostic testing, physical therapy, chiropractic care and palliative care.
The health care practice guidelines apply to all treatments provided after the
effective date of the regulation adopted by the Department of Labor, May 23,
2008, and regardless of the date of injury. The guidelines are, to the extent
permitted by the most current medical science or applicable science, based on well-documented
scientific research concerning efficacious treatment for injuries and
occupational disease. To the extent that well-documented scientific research
regarding the above is not available at the time of adoption of the guidelines,
or is not available at the time of any revision to the guidelines, the
guidelines have been and will be based upon the best available information
concerning national consensus regarding best health care practices in the
relevant health care community.
The
guidelines, to the extent practical and consistent with the Act, address
treatment of those physical conditions which occur with the greatest frequency,
or which require the most expensive treatments, for work-related injuries based
upon currently available Delaware
data.
Services
rendered by any health care provider certified pursuant to 19 Del.C.
§2322D(a) to provide treatment or services for injured employees shall be
presumed, in the absence of contrary evidence, to be reasonable and necessary
if such treatment and/or services conform to the most current version of the
Delaware health care practice guidelines.
Services
rendered outside the Guidelines and/or variation in treatment recommendations
from the Guidelines may represent acceptable medical care, be considered
reasonable and necessary treatment and, therefore, determined to be
compensable, absent evidence to the contrary, and may be payable in accordance
with the Fee Schedule and Statute, accordingly.
Services
provided by any health care provider that is not certified pursuant to 19 Del.C.
§2322D(a) shall not be presumed reasonable and necessary unless such services
are pre-authorized by the employer or insurance carrier, subject to the
exception set forth in 19 Del.C. §2322D(b).
Treatment
of conditions unrelated to the injuries sustained in an industrial accident may
be denied as unauthorized if the treatment is directed toward the
non-industrial condition, unless the treatment of the unrelated injury is
rendered necessary as a result of the industrial accident.
The
Health Care Advisory Panel and Department of Labor recognized that acceptable
medical practice may include deviations from these Guidelines, as individual
cases dictate. Therefore, these Guidelines are not relevant as evidence of a
provider's legal standard of professional care.
In accordance with the requirements of the
Act, the development of the health care guidelines has been directed by a
predominantly medical or other health professional panel, with recommendations
then made to the Health Care Advisory Panel.
The
principles summarized in this section are key to the intended implementation of
these guidelines and critical to the reader's application of the guidelines in
this document.
2.1 EDUCATION
of the patient and family, as well as the employer, insurer, policy makers and
the community should be the primary emphasis in the treatment of upper
extremity pain and disability. Currently, practitioners often think of
education last, after medications, manual therapy and surgery. Practitioners
must develop and implement an effective strategy and skills to educate
patients, employers, insurance systems, policy makers and the community as a
whole. An education-based paradigm should always start with inexpensive communication
providing reassuring information to the patient. More in-depth education
currently exists within a treatment regime employing functional restorative and
innovative programs of prevention and rehabilitation. No treatment plan is
complete without addressing issues of individual and/or group patient education
as a means of facilitating self-management of symptoms and prevention.
2.2 TREATMENT
PARAMETER DURATION Time frames for specific interventions commence once treatments
have been initiated, not on the date of injury. Obviously, duration will be
impacted by patient compliance, comorbitities and availability of services.
Clinical judgment may substantiate the need to modify the total number of
visits discussed in this document. The majority of injured workers with
Shoulder Disorders often will achieve resolution of their condition within 6 to
36 visits (Guide to Physical Therapy Practice – Second Edition). It is anticipated that most injured workers
will not require the maximum number of visits described in these
guidelines. They are designed to be a
ceiling and care extending beyond the maximum allowed visits may warrant
utilization review.
2.3 ACTIVE
INTERVENTIONS emphasizing patient responsibility, such as therapeutic
exercise and/or functional treatment, are generally emphasized over passive
modalities, especially as treatment progresses. Generally, passive
interventions are viewed as a means to facilitate progress in an active
rehabilitation program with concomitant attainment of objective functional
gains. All rehabilitation programs must incorporate “Active Interventions” no
later than three weeks after the onset of treatment. Reimbursement for passive
modalities only after the first three weeks of treatment without clear evidence
of Active Interventions will require supportive documentation.
2.4 ACTIVE
THERAPEUTIC EXERCISE PROGRAM Exercise program goals should incorporate
patient strength, endurance, flexibility, coordination, and education. This
includes functional application in vocational or community settings.
2.5 POSITIVE
PATIENT RESPONSE Positive results are defined primarily as functional gains
which can be objectively measured. Objective functional gains include, but are
not limited to, positional tolerances, range of motion, strength, endurance,
activities of daily living, cognition, and efficiency/ velocity measures which
can be quantified. Subjective reports of pain and function should be considered
and given relative weight when the pain has anatomic and physiologic
correlation. Anatomic correlation must be based on objective findings.
2.6 RE-EVALUATE
TREATMENT EVERY 3-4 WEEKS If a given treatment or modality is not producing
positive results within 3-4 weeks, the treatment should be either modified or
discontinued. Reconsideration of diagnosis should also occur in the event of
poor response to a seemingly rational intervention.
2.7 SURGICAL
INTERVENTIONS Surgery should be contemplated within the context of expected
functional outcome and not purely for the purpose of pain relief. The concept
of "cure" with respect to surgical treatment by itself is generally a
misnomer. All operative interventions must be based upon positive correlation
of clinical findings, clinical course and diagnostic tests. A comprehensive
assimilation of these factors must lead to a specific diagnosis with positive
identification of pathologic condition(s).
2.8 SIX-MONTH
TIME FRAME Since the prognosis drops precipitously for returning an injured
worker to work once he/she has been temporarily totally disabled for more than
six months, the emphasis within these guidelines is to move patients along a
continuum of care and return-to-work within a six-month time frame, whenever
possible. It is important to note that time frames may not be pertinent to
injuries which do not involve work-time loss or are not occupationally related.
2.9 RETURN-TO-WORK
Even if there is residual chronic pain, return-to-work is not necessarily
contraindicated. Return-to-work may be therapeutic, assuming the work is not
likely to aggravate the basic problem or increase long-term pain. The
practitioner must write detailed restrictions when returning a patient to
limited duty. The following functions should be considered and modified as
recommended: lifting, pushing, pulling, crouching, walking, using stairs,
bending at the waist, awkward and/or sustained postures, tolerance for sitting
or standing, hot and cold environments, data entry and other repetitive motion
tasks, sustained grip, tool usage and vibration factors. The patient should
never be released to "sedentary or light duty" without specific
physical limitations. The practitioner must understand all of the physical,
demands of the patient's job position before returning the patient to full duty
and should request clarification of the patient's job duties.
2.10 DELAYED
RECOVERY The Department recognizes that not of all industrially injured patients
will not recover within the time lines outlined in this document despite
optimal care. Such individuals may require treatments beyond the limits
discussed within this document, but such treatment will require clear
documentation by the authorized treating practitioner focusing on objective
functional gains afforded by further treatment and impact upon prognosis.
The remainder of this document should be
interpreted within the parameters of these guideline principles which will
hopefully lead to more optimal medical and functional outcomes for injured
workers.
This section addresses the shoulder and the
ten most common work-related injuries/syndromes/ disorders to or involving the
shoulder complex. The following format was developed to reduce repetitive text:
3.1 HISTORY
TAKING AND PHYSICAL EXAMINATION provides information common to all injuries
through a discussion of provider procedures which should be applied to each
patient, regardless of the injury and diagnosis (this subsection is standard to
all Division medical treatment guidelines).
3.2 SPECIFIC
DIAGNOSIS, TESTING AND TREATMENT PROCEDURES provides information unique to
each of the following work-related injuries/syndromes/disorders:
3.2.1 Acromioclavicular
(AC) Joint Sprains/Dislocations
3.2.2 Adhesive
Capsulitis/Frozen Shoulder Disorders
3.2.3 Bicipital
Tendon Disorders
3.2.4 Brachial
Plexus Injuries
3.2.4.1 Brachial Plexus
3.2.4.2 Axillary Nerve
3.2.4.3 Long Thoracic Nerve
3.2.4.4 Musculocutaneous Nerve
3.2.4.5 Spinal Accessory Nerve
3.2.4.6 Suprascapular Nerve
3.2.5 Bursitis
of the Shoulder
3.2.6 Impingement
Syndrome
3.2.7 Rotator
Cuff Tears
3.2.8 Rotator
Cuff Tendinitis
3.2.9 Shoulder
Fractures
3.2.9.1 Clavicular Fracture
3.2.9.2 Proximal Humeral Fracture
3.2.9.3 Humeral Shaft Fracture
3.2.9.4 Scapular Fracture
3.2.9.5 Sternoclavicular
Dislocation/Fracture
3.2.10 Shoulder
Instability
Each diagnosis is presented in the following format:
3.2.10.1 A
definition of the injury/disorder/syndrome;
3.2.10.2 Discussion
of relevant physical findings;
3.2.10.3 Applicable
testing and diagnostic procedures;
3.2.10.4 Diagnosis-based,
non-operative therapeutic treatment procedures;
3.2.10.5 Options
for operative/surgical treatment; and
3.2.10.6 Options
for post-operative rehabilitation/treatment procedures.
3.3 MEDICATION
provides information common to all injuries through detailed discussions of
referenced medications with indications for expected time to produce effect,
frequency, and optimum and maximum durations.
3.4 NON-OPERATIVE
TREATMENT PROCEDURES provides information common to all injuries through
detailed discussions of referenced therapeutic procedures with indications for
expected time to produce effect, frequency, and optimum and maximum durations.
As shoulder injuries frequently involve a
complex of problems, it is always necessary to consider the possible
interaction of the various parts of the shoulder mechanism when proceeding with
a diagnostic workup and a therapeutic treatment plan. Injuries to the shoulder
may require the provider to reference and/or use the other Division medical
treatment guidelines (i.e., Thoracic Outlet Syndrome Cumulative Trauma
Disorder, and/or Complex Regional Pain Syndrome/Reflex Sympathetic Dystrophy.
There are two standard procedures that
should be utilized when initially diagnosing work-related shoulder instability.
These procedures are generally accepted, well-established and widely used
procedures that establish the foundation/basis for and dictate all other
following stages of diagnostic and therapeutic procedures. When findings of
clinical evaluations and those of other diagnostic procedures are not
complementing each other, the objective clinical findings should have
preference.
4.1 HISTORY
TAKING should address at least the following for each shoulder injury
diagnosis:
4.1.1 Occupational
relationship, and
4.1.2 History
of non-occupational injury and avocational pursuits need to be specifically
documented.
4.2 PHYSICAL FINDINGS are specific to
and addressed within each shoulder injury diagnosis noted in this section.
Given the complexity of the shoulder mechanism, an evaluation for concomitant
injury should be considered.
5.1 ACROMIOCLAVICULAR
JOINT SPRAINS/DISLOCATIONS An acute acromioclavicular (AC) joint injury is
frequently referred to as a shoulder separation. There are six classifications
of an AC joint separation which are based upon the extent of ligament damage
and bony displacement:
·
Type I Partial
disruption of the AC ligament and capsule.
·
Type II Sprains
consisting of a ruptured AC ligament and capsule with incomplete injury to the
coracoclavicular (CC) ligament, resulting in minimal AC joint subluxation.
·
Type III
Separation or complete tearing of the AC ligament and/or CC ligaments, possible
deltoid trapezius fascial injury, and dislocation of the AC joint.
·
Type IV
Dislocation consisting of a displaced clavicle that penetrates posteriorly
through or into the trapezius muscle.
·
Type V
Dislocation consisting of complete separation of the AC and CC ligaments and
dislocation of the acromioclavicular joint with a large coracoclavicular
interval.
• Type VI Dislocation consisting of a displaced
clavicle that penetrates inferior to the coracoid. Types I-III are common,
while Types IV-VI are not and, when found, require surgical consultation. For
AC joint degeneration from repetitive motion
that is found to be work-related, see section 5.4.8, Impingement Syndrome.
5.1.1 History
and Initial Diagnostic Procedures (AC Joint Sprains/Dislocations):
• Occupational
Relationship - generally, workers sustain an AC joint injury when they land on
the point of the shoulder, driving the acromion downward, or fall on an
outstretched hand or elbow, creating a backward and outward force on the
shoulder. It is important to rule out other sources of shoulder pain from an
acute injury, including rotator cuff tear, fracture and nerve injury.
5.1.2 Physical
Findings (AC Joint Sprains/Dislocations) may include:
5.1.2.1 Tenderness
at the AC joint with, at times, contusions and/or abrasions at the joint area;
prominence/asymmetry of the shoulder can be seen; and/or
5.1.2.2 One
finds decreased shoulder motion and with palpation, the distal end of the
clavicle is painful; there may be increased clavicular translation; cross-body
adduction can cause exquisite pain.
5.1.3 Laboratory
Tests (AC Joint Sprains/Dislocations): are not indicated unless a systemic
illness or disease is suspected.
5.1.4 Testing
Procedures (AC Joint Sprains/Dislocations):
5.1.4.1 Plain
x-rays may include:
5.1.4.1.1 AP
view;
5.1.4.1.2 AP
radiograph of the shoulder with the beam angled 10 cephalad (Zanca view);
5.1.4.1.3 Axillary
lateral views; and
5.1.4.1.4 Y-view
also called a StrykerStyrker notch view;
5.1.4.1.5 Stress
view; side-to-side comparison with 10-15 lbs. of weight in each hand.
5.1.4.2 Adjunctive testing, such as standard
radiographic techniques (sonography, arthrography or MRI), should be considered
when shoulder pain is refractory to 4-6 weeks of non-operative conservative
treatment and the diagnosis is not readily identified by a good history and
clinical examination.
5.1.5 Non-operative
Treatment Procedures (AC Joint Sprains/Dislocations): may include:
5.1.5.1 Procedures
outlined in this Section 5.3.5 such as thermal treatment and immobilization
(up-to-6 weeks for Type I-III AC joint separations). Immobilization treatments
for Type III injuries are controversial and may range from a sling to surgery.
5.1.5.2 Medication,
such as nonsteroidal anti-inflammatories and analgesics, would be indicated;
narcotics are not normally indicated but may be needed after an acute injury.
In the face of chronic acromioclavicular joint pain, a series of injections
with or without cortisone, may be injected 6-8 times per year.
5.1.5.3 Physical medicine interventions, as
outlined in Section 5.3.5, should emphasize a progressive increase in range of
motion without exacerbation of the AC joint injury. With increasing motion and
pain control, a strengthening program should be instituted and return to
modified/limited duty would be considered at this time. By 8-11 weeks, with
restoration of full motion, return to full duty should be anticipated.
5.1.6 Operative
Procedures (AC Joint Sprains/Dislocations):
5.1.6.1 With
a Type III AC joint injury, an appropriate orthopedic consultation should be
considered initially, but must be considered when conservative care fails to
increase function.
5.1.6.2 With
a Type IV-VI AC joint injury, an orthopedic surgical consultation is
recommended initially.
5.1.7 Post-Operative
Procedures (AC Joint Sprains/Dislocations): should be coordinated by the
orthopedic physician working with the interdisciplinary team. Keeping with the
therapeutic and rehabilitation procedures found in this Section 5.3.5.
Non-operative Treatment Procedures, the patient could be immobilized for 2-3
weeks, restricted in activities, both work-related and avocational for 8-12
weeks while undergoing rehabilitation, and be expected to progress to return to
full duty based upon the his/her response to rehabilitation and the demands of
the job.
5.2 ADHESIVE
CAPSULITIS/FROZEN SHOULDER DISORDERS Adhesive capsulitis of the shoulder,
also known as frozen shoulder disorder, is a soft tissue lesion of the
glenohumeral joint resulting in restrictions of passive and active range of
motion. Occupational adhesive capsulitis arises secondarily to any chest or
upper extremity trauma. Primary adhesive capsulitis is rarely occupational in
origin. The disorder goes through stages, specifically:
·
Stage 1 Consists
of acute pain with some limitation in range of motion; generally lasting 2-9
months.
·
Stage 2
Characterized by progressive stiffness, loss of range-of-motion, and muscular
atrophy; it may last an additional 4-12 months beyond Stage 1.
·
Stage 3
Characterized by partial or complete resolution of symptoms and restoration of
range-of-motion and strength; it usually takes an additional 6-9 months beyond
Stage 2.
5.2.1 History and Initial Diagnostic Procedures
(Adhesive Capsulitis/Frozen Shoulder Disorder):
5.2.1.1 Occupational Relationship - There
should be some history of work related
injury. Often adhesive capsulitis is seen with impingement syndrome or other
shoulder disorders; refer to appropriate subsection of this guideline.
5.2.1.2 Patient will usually complain of
pain in the sub-deltoid region, but occasionally over the long head of the
biceps or radiating down the lateral aspect of the arm to the forearm. Pain is
often worse at night with difficulty sleeping on the involved side. Motion is
restricted and painful.
5.2.2 Physical
Findings (Adhesive Capsulitis/Frozen Shoulder Disorder): Restricted active
and passive glenohumeral range of motion is the primary physical finding. It
may be useful for the examiner to inject the glenohumeral joint with lidocaine
and then repeat range of motion to rule out other shoulder pathology; lack of
range of motion confirms the diagnosis. Postural changes and secondary trigger
points along with atrophy of the deltoid and supraspinatus muscles may be seen.
5.2.3 Laboratory Tests (Adhesive
Capsulitis/Frozen Shoulder Disorder): are not indicated unless systemic
illness or disease is suspected.
5.2.4 Testing
Procedures (Adhesive Capsulitis/Frozen Shoulder Disorder):
5.2.4.1 Plain
x-rays are generally not helpful except to rule out concomitant pathology.
5.2.4.2 Adjunctive
testing, such as standard radiographic techniques (sonography, arthrography or
MRI), to rule out concomitant pathology should be considered when shoulder pain
is refractory to 4-6 weeks of non-operative conservative treatment and the
diagnosis is not readily identified by a good history and clinical examination.
5.2.4.3 Arthrography
may be helpful in ruling out other pathology. Arthrography can also be
therapeutic as steroids and/or anesthetics may be injected and a brisement or
distension arthrogram can be done at the same time (refer to the next
subsection on non-operative treatment procedures for further discussion).
5.2.5 Non-operative
Treatment (Adhesive Capsulitis/Frozen Shoulder Disorder): address the goal
to restore and maintain function and may include:
5.2.5.1 A
home exercise program either alone or in conjunction with a supervised
rehabilitation program is the mainstay of treatment. Additional interventions
may include thermal treatment, ultrasound, TENS, manual therapy, and passive
and active range-of-motion exercises; as the patient progresses, strengthening
exercises should be included in the exercise regimen; refer to Section 5.3.5,
Non-operative Treatment Procedures.
5.2.5.2 Medications,
such as NSAIDs and analgesics, may be helpful. Rarely, the use of oral steroids
is indicated to decrease acute inflammation. Narcotics narcotics can be used
for short-term pain control; narcotics are indicated for post-manipulation or post-operative
cases; refer to this Section 6.0, Medications.
5.2.5.3 Occasionally,
subacromial bursal and/or glenohumeral steroid injections can decrease
inflammation and allow the therapist to progress functional exercise and range
of motion. Injections should be limited to two injections to any one site,
given at least one month apart.
5.2.5.4 In
cases that are refractory to conservative therapy lasting at least 3-6 months
and in whom range of motion remains significantly restricted (abduction less
than 90°), the following more aggressive treatment may be considered:
5.2.5.4.1 Distension
arthrography or "brisement" in which saline, an anesthetic and
usually a steroid are forcefully injected into the shoulder joint causing
disruption of the capsule. Early and aggressive physical medicine to maintain
range of motion and restore strength and function should follow distension
arthrography or manipulation under anesthesia; return to work with restrictions
should be expected within one week of the procedure; return to full duty is
expected within 4-6 weeks.
5.2.6 Operative
Procedures (Adhesive Capsulitis/Frozen Shoulder Disorder): For cases
failing conservative therapy of at least 3-6 months duration and which are
significantly limited in range-ofmotion (abduction less than 90°), the
following operative procedures may be considered:
5.2.6.1 Manipulation
under anesthesia which may be done in combination with steroid injection(s) or
distension arthrography; and
5.2.6.2 In
rare cases, refractory to conservative treatment and in which manipulation
under anesthesia is contraindicated, an open capsular release or arthroscopy
with resection of the coracohumeral and/or coracoacromial ligaments may be
done; other disorders, such as impingement syndrome, may also be treated at the
same time.
5.2.7 Post-Operative
Procedures (Adhesive Capsulitis/Frozen Shoulder Disorder): would include an
individualized rehabilitation program based upon communication between the
surgeon and the therapist.
• Early,
aggressive and frequent physical medicine interventions are recommended to
maintain range of motion and progress strengthening; return to work with
restrictions after surgery should be discussed with the treating provider;
patient should be approaching MMI within 8-12 weeks post-operative, however,
coexistence of other pathology should be taken into consideration.
5.3 BICIPITAL
TENDON DISORDERS Disorders may include 1) primary bicipital tendinitis
which is exceedingly rare; 2) secondary bicipital tendinitis which is generally
associated with rotator cuff tendinitis or impingement syndrome (see
appropriate diagnosis subsections); 3) subluxation of the biceps tendon which
occurs with dysfunction of the transverse intertubercular ligament and massive
rotator cuff tears; and 4) acute disruption of the tendon which can result from
an acute distractive force or transection of the tendon from direct trauma.
5.3.1 History
and Initial Diagnostic Procedures (Bicipital Tendon Disorders):
5.3.1.1 Occupational
Relationship - bicipital tendon disorders may include symptoms of pain and/ or
achiness that occur after repetitive use of the shoulder and/or blunt trauma to
the shoulder. Secondary bicipital tendinitis may be associated with prolonged
above-theshoulder activities, and/or repeated shoulder flexion, external
rotation and abduction. Acute trauma to the biceps tendon of the shoulder
girdle may also give rise to occupational injury of the biceps tendon.
5.3.1.2 Occupational
disorders of the biceps tendon may accompany scapulothoracic dyskinesis,
rotator cuff injury, AC joint separation, subdeltoid bursitis, shoulder
instability or other shoulder pathology. Symptoms should be exacerbated or
provoked by work that activated the biceps muscle. Symptoms may be exacerbated
by other activities that are not necessarily work related.
5.3.1.3 Symptoms
may include aching, burning and/or stabbing pain in the shoulder, usually
involving the anterior medial portion of the shoulder girdle. The symptoms are
exacerbated with above-the-shoulder activities and those specifically engaging
the biceps (flexion at the shoulder, flexion at the elbow and supination of the
forearm). Relief occurs with rest. Patients may report nocturnal symptoms which
interfere with sleep during the acute stages of inflammation; pain and weakness
in shoulder during activities; repeated snapping phenomenon with a subluxing
tendon; immediate sharp pain and tenderness along the course of the long head
of the biceps following a sudden trauma which would raise suspicions of acute
disruption of the tendon; and/or with predominant pain at the shoulder referral
patterns which may extend pain into the cervical or distal structures,
including the arm, elbow, forearm and wrist.
5.3.2 Physical
Findings (Bicipital Tendon Disorders): may include:
5.3.2.1 If
continuity of the tendon has been lost (biceps tendon rupture), inspection of
the shoulder would reveal deformity (biceps bunching);
5.3.2.2 Palpation
demonstrates tenderness along the course of the bicipital tendon;
5.3.2.3 Pain
at end range of flexion and abduction as well as biceps tendon activation;
and/or
5.3.2.4 Provocative
testing may include:
5.3.2.4.1 Yergason’s
sign - pain with resisted supination of forearm;
5.3.2.4.2 Speed's
Test - pain with resisted flexion of the shoulder (elbow extended and forearm
supinated); or
5.3.2.4.3 Ludington's
Test - pain with contraction of the biceps (hands are placed behind the head
placing the shoulders in abduction and external rotation).
5.3.3 Laboratory
Tests (Bicipital Tendon Disorders): are not indicated unless a systemic
illness or disease is suspected.
5.3.4 Testing
Procedures (Bicipital Tendon Disorders):
5.3.4.1 Plain
x-rays include:
5.3.4.1.1 Anterior/Posterior
(AP) view visualizes elevation of the humeral head, indicative of absence of
the rotator cuff due to a tear;
5.3.4.1.2 Lateral
view in the plane of the scapula and/or an axillary view determine if there is
anterior or posterior dislocation or the presence of a defect in the humeral
head (a Hill-Sachs lesion);
5.3.4.1.3 30°
caudally angulated AP view determines if there is a spur on the
anterior/inferior surface of the acromion and/or the far end of the clavicle;
and
5.3.4.1.4 Outlet
view determines if there is a downwardly tipped acromion.
5.3.4.2 Adjunctive
testing, such as sonography, MRI or arthrography, should be considered when
shoulder pain is refractory to 4-6 weeks of nonoperative conservative treatment
and the diagnosis is not readily identified by standard radiographic
techniques. These tests may be occasionally performed immediately after an
injury if tendon injury is suspected based on history and physical examination.
5.3.5 Non-operative
Treatment Procedures (Bicipital Tendon Disorders):
5.3.5.1 Benefit
may be achieved through procedures outlined in Section 5.3.5. Non-operative
Treatment Procedures, such as thermal therapy, immobilization, alteration of
occupation and/or work station, manual therapy and biofeedback.
5.3.5.2 Medication,
such as nonsteroidal anti-inflammatories and analgesics, would be indicated;
narcotics are not normally indicated but may be needed in the acute phase.
Refer to Section 5.3.5. Non-operative Treatment Procedures for further
discussions.
5.3.5.3 Physical
medicine and rehabilitation interventions, as outlined in Section 5.3.5.
Non-operative Treatment Procedures, should emphasize a progressive increase in
range of motion. With increasing motion and pain control, a strengthening
program should be instituted and return to modified/limited duty would be
considered at this time. By 8-11 weeks, with restoration of full motion, return
to full duty should be anticipated.
5.3.5.4 Biceps
tendon injections may be therapeutic if the patient responds positively to an
injection of an anesthetic. Injection of the corticosteroids directly into the
tendon should be avoided due to possible tendon breakdown and degeneration,
limited to 3 injections per year at the same site, and avoided in patients
under 30 years of age.
5.3.6 Operative
Procedures (Bicipital Tendon Disorders):
5.3.6.1 Bicipital
Tendinitis: Conservative care prior to potential surgery must address
flexibility and strength imbalances. Surgical remedies would be considered
after 12 weeks of appropriate conservative care has failed. Since impingement
of the biceps tendon could cause continued irritation, an acromioplasty may be
necessary, especially when the presence of an obstructing osteophyte is
demonstrated on plain x-rays.
5.3.6.2 Subluxing
Bicipital Tendon: The decision to surgically stabilize the bicipital tendon is
not commonly indicated. In the vast majority of cases, optimal outcome is
achieved through successful rehabilitation procedures and appropriate conservative
measures should be maximized prior to surgical intervention.
5.3.6.3 Acute
Disruption of the Bicipital Tendon: Surgical treatment shows variable
responses. Conservative care should be the mainstay of treatment with
particular attention given to the patient's age, work description and
motivation. Rarely surgery is needed to address chronic mechanical symptoms
which can occur from the intra articular residual biceps tendon stump or to
stabilize severe biceps bunching.
5.3.7 Post-Operative
Procedures (Bicipital Tendon Disorders): would include an individualized
rehabilitation program either self-directed or in a supervised setting. Rehabilitation, lasting 6-12 weeks, is often
necessary. Rehabilitation procedures discussed in Section 5.3.5, Non-operative
Treatment Procedures should be referenced and used.
5.4 BRACHIAL
PLEXUS INJURIES to the nerves and shoulder girdle region resulting in loss
of motor and sensory function, pain and instability of the shoulder. Signs and
symptoms vary with the degree of mechanism of injury. The two modes of injury
are: 1) acute direct trauma, and 2) repetitive motion or overuse. Transient
compression, stretch or traction (neuropraxia) causes sensory and motor signs
lasting days to weeks. Damage to the axon (axonomesis) without disruption of
the nerve framework may cause similar symptoms. The recovery time is delayed
and depends upon axon regrowth distally from the site of injury. Laceration or
disruption of the entire nerve with complete loss of framework (neuromesis) is
the most severe form of nerve injury. Return of function is dependent upon
regrowth of the nerve distal to the injury site.
Electromyography (EMG) is the most commonly
used diagnostic modality to analyze nerve injuries. Electrophysiologic studies,
such as electromyography and nerve conduction studies, are generally accepted,
well-established and widely used for localizing the source of neurological
symptoms. These studies should be utilized as an extension of the history and
clinical examination.
Slowing of motor nerve conduction velocities
due to demyelinization localizes regions of entrapment and injury. Denervation
demonstrated on the electromyographic portion is indicative of motor axonal or
anterior horn cell loss. Studies should be performed 3-4 weeks following injury
or description of symptoms. If the symptoms have been present for longer than
3-4 weeks, studies may be performed immediately after the initial evaluation.
Serial studies may be indicated if initial studies are negative and may also be
useful for gauging prognosis. Limb temperature should be controlled at 30-40°
centigrade. There are six relatively common nerve injuries to the shoulder
girdle; each type will be addressed separately.
5.4.1 Brachial
Plexus: is formed by the nerve roots of C5-C8 and T1; these nerve roots
exit the cervical spine and pass through the scalene musculature; after leaving
the scalene musculature, at the level of the clavicle, they form trunks,
divisions and chords which ultimately form the peripheral nerves of the arm.
5.4.1.1 History
and Initial Diagnostic Procedures (Brachial Plexus)
5.4.1.1.1 Occupational
Relationship - direct injury to brachial plexus results in widespread sensory
and motor loss. Direct trauma, subluxation to shoulder, clavicular fractures,
shoulder depression, head deviation away to the arm may result in variable
brachial plexus lesions. It is important to differentiate injuries to the
brachial plexus from the acquired (nonwork-related) syndrome of brachial plexus
neuritis, Parsonage-Turner Syndrome and/or neuralgia demyotrophy.
5.4.1.2 Physical
Findings (Brachial Plexus) may include:
5.4.1.1.2 Inspection
for evidence of trauma or deformity;
5.4.1.1.3 Identification
of sensory loss and demonstration of weakness which relates to the severity and
anatomy of the injury to the brachial plexus; and/or
5.4.1.1.4 Pain
with recreation of the motions during the mechanism of injury.
5.4.1.3 Laboratory
Tests (Brachial Plexus) are not indicated unless a systemic illness or disease
is suspected.
5.4.1.4 Testing
Procedures (Brachial Plexus) would include EMG and Nerve Conduction Studies. If
they do not localize and give sufficient information, then additional
information may be obtained from MRI and/or myelography. These studies are
employed to differentiate root avulsion from severe brachial plexus injuries.
5.4.1.5 Non-operative
Treatment Procedures (Brachial Plexus)
5.4.1.5.1 In
closed injuries, observation is favored; repeat electro physiologic studies may
be helpful to follow recovery.
5.4.1.5.2 Rehabilitation
can be utilized using procedures set forth in this Section 5.3.5, Non-operative
Treatment Procedures. However, utilization of ultrasound, cold and heat should
be discussed with the Physician since these modalities can aggravate nerve
injury.
5.4.1.5.3 Medications,
such as analgesics, nonsteroidal anti-inflammatories and anti-convulsants, are
indicated; steroids may be prescribed to help diminish the inflammatory
response, and narcotics may be indicated acutely; all medications should be
prescribed as seen in this Section 6.0, Medications.
5.4.1.6 Operative
Procedures (Brachial Plexus): In open injuries, exploration may be worthwhile
if there is poor progression of recovery from a conservative approach; in
closed injuries, if progressive weakness and loss of function is documented
after 4-6 months of conservative care, then exploration is also warranted.
5.4.1.7 Post-Operative
Procedures (Brachial Plexus) would include an individualized rehabilitation
program based upon communication between the surgeon and the therapist. This
program would begin with 4-6 weeks of rest followed by progressive increase in
motion and strength.
5.4.2 Axillary
Nerve: is derived from the 5th and 6th cervical roots; it passes around the
shoulder and supplies motor branches to the teres minor and the three heads of
the deltoid; it gives sensation to the top of the shoulder at the level of the
deltoid.
5.4.2.1 History
and Initial Diagnostic Procedures (Axillary Nerve): Occupational Relationship -
direct injury and penetrating wounds to the shoulder and upward pressure on the
axilla can cause injury to the axillary nerve; abnormalities of the nerve can
also be seen with fractures of the surgical neck of the humerus and dislocation
of the shoulder; finally, axillary nerve
injury can be seen with shoulder surgery in and of itself.
5.4.2.2 Physical
Findings (Axillary Nerve) may include:
5.4.2.2.1 Weakness
and atrophy of the deltoid muscle;
5.4.2.2.2 Strength
is lost in abduction, flexion and extension of the shoulder; and/or
5.4.2.2.3 Sensory
loss can be seen over the upper arm.
5.4.2.3 Laboratory
Tests (Axillary Nerve) are not indicated unless a systemic illness or disease
is suspected.
5.4.2.4 Testing
Procedures (Axillary Nerve) would include EMG and Nerve Conduction Studies.
5.4.2.5 Nonoperative Treatment Procedures
(Axillary Nerve)
5.4.2.5.1 Rehabilitation
can be utilized using procedures set forth in this Section 5.3.5. Non-operative
Treatment Procedures. Utilization of ultrasound, cold and heat should be
discussed with the Physician since these modalities can aggravate the nerve injury.
5.4.2.5.2 Medications
such as analgesics, nonsteroidal anti-inflammatories and anti-convulsants are
indicated and narcotics may be indicated acutely; all medications should be
prescribed as seen in this Section 6.0. Medications.
5.4.2.6 Operative
Procedures (Axillary Nerve) are usually not necessary, since most injuries to
the axillary nerve are due to stretch and/or traction. One may consider surgery
after 4-6 months with EMG/NCV documentation of ongoing denervation and loss of
function.
5.4.2.7 Post-Operative
Procedures (Axillary Nerve) would include an individualized rehabilitation
program based upon communication between the surgeon and the therapist. This
program would begin with 4-6 weeks of rest followed by progressive increase in
motion and strength.
5.4.3 Long
Thoracic Nerve: is formed by the cervical fifth, sixth, and seventh roots;
it crosses the border of the first rib and descends along the posterior surface
of the thoracic wall to the serratus anterior.
5.4.3.1 History
and Initial Diagnostic Procedures (Long Thoracic Nerve)
5.4.3.1.1 Occupational
Relationship - injury can occur by direct trauma to the posterior triangle of
the neck or trauma may be the result of chronically repeated or forceful shoulder
depression. Repeated forward motion of the arms as well as stretch or
compression of the nerve with the arms abducted can lead to long thoracic nerve
dysfunction.
5.4.3.2 Physical
Findings (Long Thoracic Nerve) may include:
5.4.3.2.1 Dull
ache in the region of the shoulder without sensory loss;
5.4.3.2.2 Scapular
deformity and/or winging may be described by patient or family; and/or
5.4.3.2.3 Serratus
Anterior (scapular winging) may be demonstrated by asking the patient to extend
and lean on his arms, such as against a wall and/or the examiner resisting
protraction.
5.4.3.3 Laboratory
Tests (Long Thoracic Nerve) are not indicated unless a systemic illness or
disease is suspected
5.4.3.4 Testing
Procedures (Long Thoracic Nerve) EMG and Nerve Conduction Studies are used to
define the anatomy and severity of the injury; side-to-side comparisons of the
nerve can be helpful to confirm the diagnosis; studies may also exclude more
widespread brachial plexus involvement.
5.4.3.5 Non-operative
Treatment (Long Thoracic Nerve)
5.4.3.5.1 Rehabilitation
can be utilized using procedures set forth in Section 5.3.5 Non-operative
Treatment Procedures. Utilization of ultrasound, cold, and heat should be
discussed with the Physician since these modalities can aggravate nerve injury.
5.4.3.5.2 Medications,
such as analgesics, nonsteroidal anti-inflammatories and anti-convulsants, are
indicated and narcotics may be indicated acutely; all medications should be
prescribed as seen in this Section 6.0 Medications.
5.4.3.6 Operative
Procedures (Long Thoracic Nerve) such as scapular fixation, may be recommended
but only in the most severe cases where there is documented significant loss of
function.
5.4.3.7 Post-Operative
Procedures (Long Thoracic Nerve) should include an individualized
rehabilitation program based upon communication between the surgeon and the
therapist. This program would begin with 8-10 weeks of rest followed by
progressive increase in motion and strength.
5.4.4 Musculocutaneous
Nerve: is derived from the fifth and sixth cervical roots; it innervates
the coracobrachialis, biceps and brachioradialis muscles and also provides
sensation to the lateral aspect of the forearm; trauma (including surgery) or
penetrating wound to the brachial plexus, coracobrachialis, and shoulder often
can cause nerve injury.
5.4.4.1 History
and Initial Diagnostic Procedures (Musculocutaneous Nerve)
5.4.4.1.1 Occupational
Relationship - most commonly a stretch/traction injury due to forceful
extension of the elbow induces nerve dysfunction; trauma can be seen to the
sensory component (lateral antebrachial cutaneous nerve) which delineates loss
of sensation to the forearm.
5.4.4.2 Physical
Findings (Musculocutaneous Nerve) may include:
·
Pain in the arm;
·
Weakness and
atrophy in the biceps and brachialis; and/or
·
Sensory loss over
the lateral aspect of the forearm; however, is not always seen.
5.4.4.3 Laboratory
Tests (Musculocutaneous Nerve) are not indicated unless a systemic illness or
disease is suspected.
5.4.4.4 Testing
Procedures (Musculocutaneous Nerve) include EMG and nerve conduction studies;
side-to-side comparisons of the motor and sensory components of the nerve may
be useful since standard norms are not always reliable.
5.4.4.5 Non-operative
Treatment Procedures (Musculocutaneous Nerve)
5.4.4.5.1 Rehabilitation
can be utilized using procedures set forth in this Section 5.3.5. Non-operative
Treatment Procedures. Utilization of ultrasound, cold, and heat should be
discussed with the Physician, since these modalities can aggravate nerve
injury.
5.4.4.5.2 Medications,
such as analgesics, nonsteroidal anti-inflammatories and anticonvulsants, are
indicated and narcotics may be indicated; all medications should be prescribed
as seen in this Section 6.5 Medications.
5.4.4.6 Operative
Procedures (Musculocutaneous Nerve) are usually not necessary unless there has
been increasing loss of function over 4-6 months and/or a laceration to the
nerve has been identified.
5.4.4.7 Post-Operative
Procedures (Musculocutaneous Nerve) would include an individualized
rehabilitation program based upon communication between the surgeon and the
therapist. This program would begin with 8-10 weeks of rest followed by
progressive increase in motion and strength.
5.4.5 Spinal
Accessory Nerve: is the eleventh cranial nerve; the nerve innervates the
ipsilateral sternocleidomastoid and trapezius muscles which are extremely
important for scapular control and ultimately shoulder function.
5.4.5.1 History
and Initial Diagnostic Procedures (Spinal Accessory Nerve)
5.4.5.1.1 Occupational
Relationship - direct trauma to the posterior neck, forceful compression of the
shoulder downward and/or deviation of the head away from the traumatized
shoulder can lead to injury to this nerve; surgical resection of the posterior
neck can disrupt the nerve.
5.4.5.2 Physical
Findings (Spinal Accessory Nerve) may include:
·
Pain in the
shoulder;
·
Weakness or
paralysis of the trapezius which is seen as winging with the arms out to the
side (abduction); and/or
·
Drooping of the
shoulder.
5.4.5.3 Laboratory
Tests (Spinal Accessory Nerve) are not indicated unless a systemic illness or
disease is suspected.
5.4.5.4 Testing
Procedures (Spinal Accessory Nerve) include EMG and Nerve Conduction Studies
are used to define the anatomy and severity of the injury; side-to-side
comparisons of the nerve can be helpful to confirm the diagnosis; radiographic
procedures may be necessary to exclude lesion at the base of the brain or upper
cervical spine.
5.4.5.5 Non-operative
Treatment Procedures (Spinal Accessory Nerve)
5.4.5.5.1 Rehabilitation
can be utilized using procedures set forth in Section 5.3.5. Non-operative
Treatment Procedures. Utilization of ultrasound, cold, and heat should be
discussed with the Physician, since these modalities can aggravate nerve
injury.
5.4.5.5.2 Medications,
such as analgesics, nonsteroidal anti-inflammatories and anticonvulsants, are
indicated and narcotics may be indicated acutely; all medications should be
prescribed as seen in Section 6.5 Medications.
5.4.5.6 Operative Procedures (Spinal Accessory
Nerve) are usually not necessary unless increased loss of function over 4-6
months has been documented and/or a laceration to the nerve has been
identified.
5.4.5.7 Post-Operative
Procedures (Spinal Accessory Nerve) would include an individualized
rehabilitation program based upon communications between the surgeon and the therapist.
This program would begin with 8-10 weeks of rest followed by progressive
increase in motion and strength.
5.4.6 Suprascapular
Nerve: is derived from the fifth and sixth cervical root, superior trunk of
the brachial plexus, and it innervates the supraspinatus and infraspinatus
muscles of the rotator cuff.
5.4.6.1 History
and Initial Diagnostic Procedures (Suprascapular Nerve)
5.4.6.1.1 Occupational
Relationship - supraclavicular trauma, stretch, and friction through the
suprascapular notch or against the transverse ligament at the notch can cause
injury to the nerve; repetitive use of the arm has been shown on occasion to
cause traction to the nerve.
5.4.6.2 Physical
Findings (Suprascapular Nerve) may include:
·
Pain at the
shoulder;
·
Wasting at the
supraspinatus and/or infraspinatus muscles with weakness; and/or
·
Tinel's can help
to elicit a provocative pain response.
5.4.6.3 Laboratory
Tests (Suprascapular Nerve) are not indicated unless a systemic illness or
disease is suspected.
5.4.6.4 Testing
Procedures (Suprascapular Nerve) include EMG and nerve conduction studies;
side-to-side comparisons may be useful since standard norms are not always
reliable. If one suspects a mass lesion at the suprascapular notch, then an MRI
may be indicated.
5.4.6.5 Non-operative
Treatment Procedures (Suprascapular Nerve)
5.4.6.5.1 Rehabilitation
can be utilized using procedures set forth in Section 5.3.5. Non-operative
Treatment Procedures. Utilization of ultrasound, cold, and heat should be
discussed with the Physician, since these modalities can aggravate nerve
injury.
5.4.6.5.2 Medications,
such as analgesics, nonsteroidal anti-inflammatories and anti-convulsants, are
indicated and narcotics may be indicated acutely; all medications should be
prescribed as seen in this Section 6.5 Medications.
5.4.6.6 Operative
Treatment Procedures (Suprascapular Nerve) involving surgical release at the suprascapular
notch or spinoglenoid region is warranted depending upon the results of the
electrophysiologic studies and/or absence of improvement with conservative
management.
5.4.6.7 Post-Operative
Procedures (Suprascapular Nerve) would include an individualized rehabilitation
program based upon communication between the surgeon and the therapist. This
program would begin with 8-10 weeks of rest followed by progressive increase in
motion and strength.
5.5 BURSITIS OF THE
SHOULDER Acute or chronic inflammation of the bursa (a potential fluid
filled sac) that may be caused by trauma, chronic overuse, inflammatory
arthritis, and acute or chronic infection that generally presents with
localized pain and tenderness of the shoulder.
5.5.1 History and Initial Diagnostic
Procedures (Bursitis of the Shoulder):
·
Occupational
Relationship -onset of symptoms, date, mechanism of onset, and occupational
history and current requirements should be correlated with the intensity,
character, duration and frequency of associated pain and discomfort.
·
History may
include nocturnal pain, pain with over-the-shoulder activities, feeling of
shoulder weakness, prior treatment for presenting complaint(s), specific
limitations of movement and pertinent familial history.
5.5.2 Physical
Findings (Bursitis of the Shoulder): may include:
·
Palpation elicits
localized tenderness over the particular bursa or inflamed tendon; loss of
motion during activity;
·
Painful arc may
be seen between 40-120° and/or
·
Bursitis may be
associated with other shoulder injury diagnoses such as impingement, rotator
cuff instability, tendonitis, etc.; refer to applicable diagnosis subsections
for additional guidelines.
5.5.3 Laboratory
Tests (Bursitis of the Shoulder): may be used to rule out systemic illness
or disease when proper clinical presentation indicates the necessity for such
testing. Testing could include sedimentation rate, rheumatoid profile, complete
blood count (CBC) with differential, serum uric acid level, routine screening
of other medical disorders may be necessary, as well as bursal aspiration with
fluid analysis.
5.5.4 Testing
Procedures (Bursitis of the Shoulder):
5.5.4.1 Plain x-rays include:
5.5.4.1.1 AP view visualizes elevation of the humeral
head, indicative of absence of the rotator cuff due to a tear;
5.5.4.1.2 Lateral
view in the plane of the scapula or an axillary view determines if there is
anterior or posterior dislocation or the presence of a defect in the humeral
head (a Hill-Sachs lesion);
5.5.4.1.3 30° caudally angulated AP view determines if
there is a spur on the anterior/ interior surface of the acromion and/or the
far end of the clavicle; and
5.5.4.1.4 Outlet
view determines if there is a downwardly tipped acromion.
5.5.4.2 Adjunctive
testing, such as standard radiographic techniques (sonography, arthrography or
MRI), should be considered when shoulder pain is refractory to 4-6 weeks of
non-operative conservative treatment and the diagnosis is not readily
identified by a good history and clinical examination.
5.5.5 Non-operative
Treatment Procedures (Bursitis of the Shoulder):
5.5.5.1 Benefits
may be achieved through procedures outlined in Section 5.3.5. Non-operative
Treatment Procedures, such as immobilization, therapeutic exercise, alteration
of occupation and work station, thermal therapy, TENS unit, and ultrasound.
5.5.5.2 May
return to work without overhead activities and lifting with involved arm until
cleared by physician for those and heavier activities.
5.5.5.3 Additional
modalities/treatment procedures may include biofeedback; physical medicine and
rehabilitation including instruction in therapeutic exercise, proper work
technique and manual therapy; vocational rehabilitation, vocational assessment
and interdisciplinary team approach.
5.5.5.4 Medications such as nonsteroidal
anti-inflammatories and analgesics. Subacromial space injection may be
therapeutic but should be limited to 3 injections per year in the same location.
Injection of the corticosteroids directly into the tendons should be avoided
due to possible tendon breakdown and degeneration. There are rare occasions
where intratendinous injections may be cautiously considered if calcific
tendonitis is present.
Rarely are injections used in patients under
30 years of age.
5.5.6 Operative
Procedures (Bursitis of the Shoulder): are not commonly indicated for pure
bursitis; refer to other appropriate diagnoses in Section 5.0. Specific
Diagnosis, Testing and Treatment Procedures.
5.6 IMPINGEMENT
SYNDROME A collection of symptoms, not a pathologic diagnosis. The symptoms
result from the encroachment of the acromion, coracoacromial ligament, coracoid
process, and/or the AC joint of the rotator cuff mechanism that passes beneath
them as the shoulder is moved. The cuff mechanism is intimately related to the
coracoacromial arch. Separated only by the thin lubricating surfaces of the
bursa, compression and friction can be minimized by several factors, such as
·
Shape of the
coracoacromial arch that allows passage of the subjacent rotator cuff;
·
Normal
undersurface of the AC Joint;
·
Normal bursa;
·
Normal capsular
laxity; and
·
Coordinated
scapulothoracic function.
The
impingement syndrome may be associated with AC joint arthritis, both partial-
and full-thickness rotator cuff tears, adhesive capsulitis/frozen shoulder and
bursitis. Normal function of the rotator cuff mechanism and biceps tendon
assist to diminish impingement syndrome.
5.6.1 History and Initial
Diagnostic Procedures (Impingement Syndrome):
5.6.1.1 Occupational
Relationship -established repetitive overuse of the upper extremity; many times
this is seen with constant overhead motion.
5.6.1.2 History
may include:
5.6.1.2.1 Delayed
presentation; since the syndrome is usually not an acute problem; patients will
access care if their symptoms have not resolved with rest, time and
"trying to work it out";
5.6.1.2.2 Complaints
of functional losses due to pain, stiffness, weakness and catching when the arm
is flexed and internally rotated; and
5.6.1.2.3 Poor
sleep is common and pain is often felt down the lateral aspect of the upper arm
near the deltoid insertion or over the anterior proximal humerus.
5.6.2 Physical
Findings (Impingement Syndrome): may include:
5.6.2.1 Inspection
of the shoulder may reveal deltoid and rotator cuff atrophy;
5.6.2.2 Range
of motion is limited particularly in internal rotation and in cross-body
adduction;
5.6.2.3 Passive
motion through the 60-90° arc of flexion may be accompanied by pain and
crepitus; this is accentuated as the shoulder is moved in-and-out of internal
rotation;
5.6.2.4 Active
elevation of the shoulder is usually more uncomfortable than passive elevation;
5.6.2.5 Pain
on maximum active forward flexion is frequently seen with impingement syndrome,
but is not specific for diagnosis;
5.6.2.6 Strength
testing may reveal weakness of flexion and external rotation in the scapular
plane; this weakness may be the result of disuse, tendon damage, or poor
scapulothoracic mechanics;
5.6.2.7 Pain
on resisted abduction or external rotation may also indicate that The integrity
of the rotator cuff tendons may be compromised; and/or
5.6.2.8 Weakness
of the posterior scapular stabilizers can also be seen as a contributing factor
to impingement syndrome by altering the mechanics of the glenohumeral joint.
5.6.3 Laboratory Tests
(Impingement Syndrome): are not indicated unless a systemic illness or
disease is suspected.
5.6.4 Testing Procedures
(Impingement Syndrome):
5.6.4.1
Plain x-rays include:
5.6.4.1.1 AP
view visualizes elevation of the humeral head, indicative of rotator cuff fiber
failure with diminished space at the subacromial area;
5.6.4.1.2 Lateral
view in the plane of the scapula or an axillary view can help to determine
aspects of instability which can give symptoms similar to impingement syndrome;
5.6.4.1.3 30°
caudally angulated AP view can assess for a spur on the anterior/inferior
surface of the acromion and/or the distal end of the clavicle which can lead to
encroachment on the rotator cuff mechanism with motion; and
5.6.4.1.4 Outlet
view determines if there is a downwardly tipped acromion.
5.6.4.2 Adjunctive
testing, such as standard radiographic techniques (sonography, arthrography or
MRI), should be considered when shoulder pain is refractory to 4-6 weeks of
non-operative conservative treatment and the diagnosis is not readily
identified by a good history and clinical examination.
5.6.5 Non-operative Treatment
Procedures (Impingement Syndrome) may include:
5.6.5.1 Medications,
such as nonsteroidal anti-inflammatories and analgesics, should be prescribed
as seen in Section 6.5 Medications. Subacromial space injection may be
therapeutic. Injections of corticosteroids into the subacromial space should be
limited to 3 injections per year at the same site, and rarely used in patients
less than 30 years.
5.6.5.2 In
order to have the most favorable outcome from a conservative approach, an
aggressive attempt should be made to define the contributing factors which are
driving the syndrome, such as shoulder stiffness, humeral head depressor
weakness (rotator cuff fiber failure), and subacromial crowding AC Joint
arthritis.
5.6.5.3 Procedures
outlined in Section 5.3.5. Non-operative Treatment Procedures should be
considered, such as relative rest, immobilization, thermal treatment,
ultrasound, therapeutic exercise and physical medicine and rehabilitation.
5.6.6 Operative
Procedures (Impingement Syndrome): should restore functional anatomy by
reducing the potential for repeated impingement; procedures might include
distal clavicular resection, coracoacromial ligament release, and/or
acromioplasty.
5.6.7 Post-Operative
Procedures (Impingement Syndrome): would include an individualized
rehabilitation program based upon communication between the surgeon and the
therapist.
5.6.7.1 Individualized
rehabilitation programs might include:
5.6.7.1.1 Sling
or abduction splint;
5.6.7.1.2 Gentle
pendulum exercise, passive glenohumeral range of motion and aggressive
posterior scapular stabilizing training can be instituted;
5.6.7.1.3 At
4 weeks post-operative, begin isometrics and ADL involvement; and/or
5.6.7.1.4 Depending
upon the patient's functional response, at 4 weeks post-operative consider
beginning light resistive exercise; concomitantly, return to a light modified
duty may be plausible given the ability to accommodate "no repetitive
overhead activities."
5.6.7.2 Progressive
resistive exercise from 2 months with gradual returning to full activity at 5-7
months; all active non-operative procedures listed in this Section 5.3.5.
Non-operative Treatment Procedures should be considered.
5.6.7.3 Work
restrictions should be evaluated every 4-6 weeks during post-operative recovery
and rehabilitation with appropriate written communications to both the patient
and the employer. Should progress plateau, the provider should reevaluate the
patient's condition and make appropriate adjustments to the treatment plan.
5.7 ROTATOR
CUFF TEAR Partial- or full-thickness tears of the rotator cuff tendons,
most often the supraspinatus can be caused by vascular, traumatic or
degenerative factors or a combination. Further tear classification includes: a
small tear is less than 1cm; medium tear is 1-3cm; large tear is 3-5cm; and
massive tear is greater than 5cm, usually with retraction.
5.7.1 History
and Initial Diagnostic Procedures (Rotator Cuff Tear):
5.7.1.1 Occupational
Relationship - established with sudden trauma to the shoulder or chronic
over-use with repetitive overhead motion with internal or external rotation.
5.7.1.2 History
may include:
5.7.1.2.1 Partial-thickness cuff tears usually occur in
age groups older than 30. Full-thickness tears can occur in younger age groups.
5.7.1.2.2 Complaints
of pain along anterior, lateral or posterior glenohumeral joint.
5.7.2 Physical Findings (Rotator Cuff Tear) may include:
5.7.2.1 Partial-Thickness
Tear
5.7.2.1.1 There will be pain at the end of range of
motion with full passive range-of-motion for abduction, elevation, external
rotation; internal rotation is attainable;
5.7.2.1.2 Active range of motion will be limited and
painful for abduction and external rotation, as well as internal rotation and
forward flexion;
5.7.2.1.3 A
painful arc may be present with active elevation;
5.7.2.1.4 Pain
will be positive for resisted tests (abduction, flexion, external rotation,
internal rotation, abduction/internal rotation at 90°, and abduction/external
rotation at 45°; and/or
5.7.2.1.5 If
there are positive impingement signs, see this Section 5.4.8, Impingement
Syndrome.
5.7.2.2 Full-Thickness Tears
5.7.2.2.1 Passive and resisted findings are similar to
those for partial-thickness tears; and /or
5.7.2.2.2 Active
elevation will be severely limited with substitution of scapular rotation being
evident.
5.7.3 Laboratory
Tests (Rotator Cuff Tear): are not indicated unless a systemic illness or
disease is suspected.
5.7.4 Testing
Procedures (Rotator Cuff Tear):
5.7.4.1 Plain
x-rays include:
5.7.4.1.1 AP view visualizes elevation of the humeral
head, indicative of absence of the rotator cuff due to a tear;
5.7.4.1.2 Lateral view in the plane of the scapula
and/or an axillary view determines if there is anterior or posterior
dislocation or the presence of a defect in the humeral head (a Hill-Sachs
lesion);
5.7.4.1.3 30°
caudally angulated AP view determines if there is a spur on the anterior
inferior surface of the acromion and/or the far end of the clavicle; and
5.7.4.1.4 Outlet
view determines if there is a downwardly tipped acromion.
5.7.4.2 Adjunctive
testing should be considered when shoulder pain is refractory to 4-6 weeks of
non-operative conservative treatment and the diagnosis is not readily
identified by standard radiographic techniques, then sonography, arthrography
or MRI may be indicated. These tests may be occasionally performed immediately
after an injury if rotator cuff tear is suspected based on history and physical
exam.
5.7.5 Non-operative
Treatment Procedures (Rotator Cuff Tear):
5.7.5.1 Medications,
such as nonsteroidal anti-inflammatories and analgesics, would be indicated;
acute rotator cuff tear could indicate the need for limited narcotics use.
5.7.5.2 Relative
rest and procedures outlined in Section 5.3.5. Non-operative Treatment
Procedures, such as immobilization, therapeutic exercise, alteration of
occupation/work station, thermal treatment, TENS unit, therapeutic ultrasound,
return-to-work, biofeedback and physical medicine and rehabilitation. If no
increase in function for a partial- or full-thickness tear is observed after
6-8 weeks, a surgical consultation is indicated. Early surgical intervention
produces better surgical outcome due to healthier tissues and often less
limitation of movement prior to and after surgery.
5.7.6 Operative
Procedures (Rotator Cuff Tear): options would include arthroscopic repair
or an open debridement and repair. Goals of surgical intervention are to restore
functional anatomy by reestablishing continuity of the rotator cuff, and to
reduce the potential for repeated impingement by the performance of procedures
such as distal clavicular resection, coracoacromial ligament release, and/or
anterior acromioplasty (subacromial decompression).
5.7.7 Post-Operative
Procedures (Rotator Cuff Tear): would include an individualized
rehabilitation program either home based or in conjunction with supervised
therapy.
5.7.7.1 Individualized
rehabilitation program might include:
·
Sling or
abduction splint;
·
Gentle pendulum
exercise, passive glenohumeral range of motion in flexion and external rotation
to prevent adhesions and maintain mobilization with or without the assistance
of a pulley;
·
At 4 to 6 weeks
post-operative begin isometrics and ADL involvement;
·
Active assisted
range-of-motion in supine with progression to sitting;
·
At 6-8 weeks,
depending on quality of tissue, begin light resistive exercise;
·
Pool exercise, manual
resistive exercise to 90°, scapula mobilization exercise with glenohumeral
stabilization; and
·
Scapular plane
exercise.
5.7.7.2 Progressive
resistive exercise from 3-6 months, with gradual returning to full activity at
6-9 months. All active non-operative procedures listed in this Section 5.3.5.
Non-operative Treatment Procedures should be considered.
5.7.7.3 Work
restrictions should be evaluated every 4-6 weeks during post-operative recovery
and rehabilitation with appropriate written communications to both the patient
and employer. Should progress plateau, the provider should reevaluate the
patient's condition and make appropriate adjustments to the treatment plan.
5.8 ROTATOR
CUFF TENDINITIS Inflammation of one or more of the four musculotendinous
structures which arise from the scapula and insert on the lesser or greater
tuberosity of the humerus. These structures include one internal rotator
(subscapularis), and two external rotators (infraspinatus and teres minor), and
the supraspinatus which assists in abduction.
5.8.1 History
and Initial Diagnostic Procedures (Rotator Cuff Tendinitis):
• Occupational
Relationship - may include symptoms of pain and/or achiness that occur after
repetitive use of the shoulder and/or blunt trauma to the shoulder.
5.8.2 Physical
Findings (Rotator Cuff Tendinitis) may include:
5.8.2.1 Pain
with palpation to the shoulder with active or passive abduction and external
rotation of the shoulder (painful arc);
5.8.2.2 Pain
with impingement signs; and/or
5.8.2.3 Pain
with specific activation of the involved muscles.
5.8.3 Laboratory
Tests (Rotator Cuff Tendinitis): are not indicated unless a systemic
illness or disease is suspected.
5.8.4 Testing
Procedures (Rotator Cuff Tendinitis) may include:
5.8.4.1 Plain
x-ray films including AP lateral, axillary, 30° caudally angulated AP, and
Outlet view.
5.8.4.2 If
shoulder pain is refractory to 4-6 weeks of non-operative care and the
diagnosis is not readily identified by standard radiographic techniques, then
adjunctive testing, such as MRI, sonography or arthrography, may be indicated.
5.8.4.3 Subacromial
space injection can be used as a diagnostic procedure by injecting an
anesthesia, such as sensorcaine or xylocaine solutions, into the space. If the
pain is alleviated with the injection the diagnosis is confirmed.
5.8.5 Non-operative
Treatment Procedures (Rotator Cuff Tendinitis) may include:
5.8.5.1 Medications,
such as nonsteroidal anti-inflammatories and analgesics: Subacromial space
injection may be therapeutic. Injections of corticosteroids into the
subacromial space should be limited to 3 injections per year, rarely used in
patients less than 30 years, and generally not injected into the tendon.
Autologous blood product injections into areas of tendinopathy are an evolving
treatment and may rarely be considered.
5.8.5.2 Procedures
outlined in Section 5.3.5. Non-operative Treatment Procedures such as relative
rest, immobilization, thermal treatment, ultrasound, therapeutic exercise,
physical medicine and rehabilitation.
5.8.6 Operative
Procedures (Rotator Cuff Tendinitis): are indicated after failure of
conservative care. Surgical treatment and post operative care are similar to
the surgical treatment of shoulder bursitis and impingement syndrome. See
Sections 5.4.7 and 5.4.8.
5.9 SHOULDER
FRACTURES There are five common types of shoulder fractures; each type will
be addressed separately and in the order of most frequent occurrence.
5.9.1 Clavicular
Fracture:
5.9.1.1 History
and Initial Diagnostic Procedures (Clavicular Fracture)
• Occupational Relationship - can result from direct blows or
axial loads applied to the upper limb; commonly associated injuries include rib
fractures, long-bone fractures of the ipsilateral limb and scapulothoracic
dislocations.
5.9.1.2 Physical Findings (Clavicular Fracture)
may include:
|
5.9.1.2.1
|
Pain in the clavicle;
|
|
5.9.1.2.2
|
Abrasions on the chest wall,
clavicle and shoulder can be seen;
|
|
5.9.1.2.3
|
Deformities can be seen in
the above regions; and/or
|
|
5.9.1.2.4
|
Pain with palpation and
motion at the shoulder joint area.
|
5.9.1.3 Laboratory Tests (Clavicular Fracture) are
not indicated unless a systemic illness or disease is suspected.
5.9.1.4 Testing Procedures (Clavicular Fracture)
could include routine chest x-rays. Alternatively x-rays centered on the
clavicle, both straight AP and 20 degree cephalad AP views, would be indicated.
Serial x-rays should be performed to document healing.
5.9.1.5 Non-operative Treatment Procedures
(Clavicular Fracture)
5.9.1.5.1 Most are adequately managed by closed
techniques and do not require surgery. The arm is immobilized in a sling
(figure-8 bracing shows limited success and should be used rarely). Shoulder
rehabilitation is begun with pendulum exercises 10-14 days after injury.
Subsequently, with pain control, the therapy program can be progressed with
therapeutic approaches as seen in this Section 5.3.5. Non-operative Treatment
Procedures.
5.9.1.5.2 Medication, such as analgesics and
nonsteroidal anti-inflammatories, would be indicated; narcotics may be
indicated acutely for fracture and should be prescribed as indicated use is
indicated in Section 6.5 Medications.
5.9.1.6 Operative Procedures (Clavicular Fracture)
would be indicated for open fractures, significantly displaced fractures,
vascular or neural injuries requiring repair, bilateral fractures, ipsilateral
scapular or glenoid neck fractures, scapulothoracic dislocations, flail chest
and nonunion displaced-closed fractures that show no evidence of union after
4-6 months. Also a Type II fracture/dislocation at the AC joint where the distal
clavicular fragment remains with the acromion and the coracoid, and the large
proximal fragment is displaced upwards.
5.9.1.7 Post-Operative Procedures (Clavicular
Fracture) would include an individualized rehabilitation program. This program
would begin with 2-4 weeks of rest with a shoulder immobilizer or sling while
encouraging isometric deltoid strengthening; pendulum exercises with
progression to assisted forward flexion and external rotation would follow;
strengthening exercises should be started at 10-12 weeks as seen in Section
5.3.5. Non-operative Treatment Procedures.
5.9.2 Proximal Humeral Fractures:
5.9.2.1 History
and Initial Diagnostic Procedures (Proximal Humeral Fractures)
5.9.2.1.1 Occupational
Relationship - may be caused by a fall onto an abducted arm; may also be caused
by high-energy (velocity or crush) trauma with an abducted or non-abducted arm;
associated injuries are common, such as glenohumeral dislocation, stretch
injuries to the axillary, musculocutaneous, and radial nerves; axillary artery
injuries with high energy accident.
5.9.2.1.2 Physical
Findings (Proximal Humeral Fractures) may include:
5.9.2.1.2.1 Pain in the upper arm;
5.9.2.1.2.2 Swelling and bruising in the upper arm, shoulder and chest
wall;
5.9.2.1.2.3 Abrasions about the shoulder; and/or
5.9.2.1.2.4 Pain with any attempted passive or active shoulder motion.
5.9.2.1.3 Laboratory
Tests (Proximal Humeral Fractures) are not indicated unless a systemic illness
or disease is suspected.
5.9.2.1.4 Testing
Procedures (Proximal Humeral Fracture)
5.9.2.1.4.1 X-ray
trauma series (3 views) are needed; AP view, axillary view and a lateral view
in the plane of the scapula. Additionally, AP view may be done in externally
rotation and also internal rotation
5.9.2.1.4.2 Vascular
studies are obtained emergently if the radial and brachial pulses are absent.
5.9.2.1.4.3 Diagnostic
testing including CT Scan or MRI to further evaluate the fracture and
surrounding structures may be appropriate depending on the fracture
configuration and need for pre-operative planning.
5.9.2.1.5 Non-operative
Treatment Procedures (Proximal Humeral Fractures)
5.9.2.1.5.1 Impacted
or minimally displaced fractures of the humeral neck or greater tuberosity are
generally managed non-operatively.
5.9.2.1.5.2 Isolated
and minimally displaced (less than 1cm) fractures are treated non operatively.
5.9.2.1.5.3 Anterior
or posterior dislocation associated with minimally displaced fractures can
usually be reduced by closed means, but an anesthetic is needed.
5.9.2.1.5.4 Immobilization
is provided with a sling, to support the elbow, and/or an abduction immobilizer
if appropriate for the fracture configuration.
5.9.2.1.5.5 Immobilization
is continued for 4-6 weeks
5.9.2.1.5.6 Shoulder
rehabilitation is begun with pendulum exercises 10-14 days after injury.
Subsequently, with pain control, the therapy program can be progressed with
therapeutic approaches as seen in Section 5.3.5. Non-operative Treatment
Procedures.
5.9.2.1.6 Operative
Procedures (Proximal Humeral Fractures)
5.9.2.1.6.1 Indications
for operative treatment would include:
|
5.9.2.1.6.1.1
|
Unstable surgical neck
fractures (no contact between the fracture
|
|
|
fragments).
|
|
5.9.2.1.6.1.2
|
Partially unstable fractures
(only partial contact) with associated same
|
|
|
upper extremity injuries.
|
|
5.9.2.1.6.1.3
|
Displaced 3- and 4-part
fractures may be managed by internal fixation or
|
|
|
a prosthetic hemiarthroplasty
and reattachment of the tuberosities.
|
5.9.2.1.7 Post-Operative Procedures (Proximal Humeral
Fractures) would include an individualized rehabilitation program.
5.9.2.1.7.1 See this Section 5.4.11, Shoulder
Fracture, Non-operative Treatment Procedures.
5.9.3 Humeral Shaft
Fractures:
5.9.3.1 History
and Initial Diagnostic Procedures (Humeral Shaft Fractures)
• Occupational
Relationship - a direct blow can fracture the humeral shaft at the junction of
its middle and distal thirds; twisting injuries to the arm will cause a spiral
humeral shaft fracture; high energy (velocity or crush) will cause a comminuted
humeral shaft fracture.
5.9.3.2 Physical
Findings (Humeral Shaft Fractures) may include:
5.9.3.2.1 Deformity
of the arm;
5.9.3.2.2 Bruising
and swelling; and/or
5.9.3.2.3 Possible
sensory and/or motor dysfunction of the radial nerve.
5.9.3.3 Laboratory
Tests (Humeral Shaft Fractures) are not indicated unless a systemic illness or
disease is suspected.
5.9.3.4 Testing
Procedures (Humeral Shaft Fractures)
5.9.3.4.1 Plain
x-rays including AP view and lateral of the entire humeral shaft.
5.9.3.4.2 Vascular
studies if the radial pulse is absent.
5.9.3.4.3 Compartment
pressure measurements if the surrounding muscles are swollen, tense and painful
and particularly if the fracture resulted from a crush injury.
5.9.3.5 Non-operative
Treatment Procedures (Humeral Shaft Fractures)
5.9.3.5.1 Most
isolated humeral shaft fractures can be managed non-operatively.
5.9.3.5.2 A
coaptation splint may be applied. The splint is started in the axilla, extended
around the elbow and brought up to the level of the acromion. It is held in
place with large elastic bandages.
5.9.3.5.3 At
2-3 weeks after injury, a humeral fracture orthosis may be used to allow for
full elbow motion.
5.9.3.6 Operative
Treatment (Humeral Shaft Fractures)
5.9.3.6.1 Indications
for operative care would include:
·
Open fracture;
·
Associated
forearm or elbow fracture (i.e., the floating elbow injury);
·
Burned upper
extremity;
·
Associated
paraplegia;
·
Multiple injuries
(polytrauma);
·
A radial nerve
palsy which came on after closed reduction; and/or
·
Pathologic
fracture related to an occupational injury.
·
Some instable or
significantly displaced fractures
5.9.3.6.2 Accepted
methods of internal fixation include:
5.9.3.6.2.1 A
broad plate and screws; and/or
5.9.3.6.2.2 Intramedullary
rodding with or without cross-locking screws.
5.9.3.7 Post-Operative
Procedures (Humeral Shaft Fractures) would include an individualized
rehabilitation program. Following rigid internal fixation, therapy may be
started to obtain passive and later active shoulder motion using appropriate
therapeutic approaches as seen in Section 5.3.5. Non-operative Treatment
Procedures. Active elbow and wrist motion may be started immediately.
5.9.4 Scapular Fractures:
5.9.4.1 History
and Initial Diagnostic Procedures (Scapular Fractures)
• Occupational
Relationship - these are the least common of the fractures about the shoulder
and include acromial, glenoid, glenoid neck and scapular body fractures. With
the exception of anterior glenoid lip fractures caused by an anterior shoulder
dislocation, all other scapular fractures are due to a high energy injury.
5.9.4.2 Physical
Findings (Scapular Fractures) may include:
5.9.4.2.1 Pain
about the shoulder and thorax;
5.9.4.2.2 Bruising
and abrasions;
5.9.4.2.3 Possibility
of associated humeral or rib fractures; and/or
5.9.4.2.4 Vascular
problems (pulse evaluation and Doppler examination).
5.9.4.3 Laboratory
Tests (Scapular Fractures), because of the association of high energy trauma,
may include a complete blood count, urinalysis and chest x-ray are warranted.
5.9.4.4 Testing
Procedures (Scapular Fractures)
5.9.4.4.1 Trauma
x-ray series - AP view, axillary view and a lateral view in the plane of the
scapula.
5.9.4.4.2 Arteriography
if a vascular injury is suspected.
5.9.4.4.3 Electromyographic
exam if nerve injuries are noted.
5.9.4.4.4 Diagnostic
testing including CT Scan or MRI to evaluate fracture and surrounding
structures.
5.9.4.5 Non-operative
Treatment Procedures (Scapular Fractures)
5.9.4.5.1 Non-displaced
acromial, coracoid, glenoid, glenoid neck and scapular body fractures may all
be treated with the use of a shoulder immobilizer.
5.9.4.5.2 Pendulum
exercises may be started within the first week.
5.9.4.5.3 Progress
to assisted range of motion exercises at 3-4 weeks using appropriate
therapeutic procedures as seen in this Section 5.3.5. Non-operative Treatment
Procedures.
5.9.4.6 Operative
Treatment (Scapular Fractures)
5.9.4.6.1 Acromial
fractures which are displaced should be internally fixed to prevent a nonunion.
These fractures may be fixed with lag screws and/or a superiorly placed plate
to neutralize the muscular forces.
5.9.4.6.2 Glenoid
fractures which are displaced greater than 2-3 mm should be fixed internally.
The approach is determined by studying the results of a CT scan.
5.9.4.6.3 Scapular
body fractures require internal fixation if the lateral or medial borders are
displaced to such a degree as to interfere with scapulothoracic motion.
5.9.4.6.4 Displaced
fractures of the scapular neck and the ipsilateral clavicle require internal
fixation of the clavicle to reduce the scapular neck fracture.
5.9.4.7 Post-Operative
Treatment (Scapular Fractures) would include an individualized rehabilitation
program Non-operative Treatment Procedures, a shoulder immobilizer is utilized,
pendulum exercises at one week, deltoid isometric exercises are started early,
and, at 4-6 weeks, active range of motion is commenced.
5.9.5 Sternoclavicular Dislocation/Fracture:
5.9.5.1 History
and Initial Diagnostic Procedures (Sternoclavicular Dislocation/Fracture)
• Occupational
Relationship -established with sudden trauma to the shoulder/ anterior chest
wall; anterior dislocations of the sternoclavicular joint usually do not
require active treatment; however, symptomatic posterior dislocations will
require reduction.
5.9.5.2 Physical Findings (Sternoclavicular
Dislocation/Fracture) may include:
5.9.5.2.1 Pain
at the sternoclavicular area;
5.9.5.2.2 Abrasions
on the chest wall, clavicle and shoulder can be seen;
5.9.5.2.3 Deformities
can be seen in the above regions; and/or
5.9.5.2.4 Pain
with palpation and motion at the sternoclavicular joint area.
5.9.5.3 Laboratory
Tests (Sternoclavicular Dislocation/Fracture) are not indicated unless a
systemic illness or disease is suspected.
5.9.5.4 Testing
Procedures (Sternoclavicular Dislocation/Fracture)
5.9.5.4.1 Plain
x-rays of the sternoclavicular joint are routinely done. When indicated,
comparative views of the contralateral limb may be necessary.
5.9.5.4.2 X-rays
of other shoulder areas and chest wall may be done if clinically indicated.
5.9.5.4.3 Vascular
studies should be considered if the history and clinical examination indicate
extensive injury.
5.9.5.4.4 Diagnostic
tests such as CT Scan or MRI may be required to fully delineate the nature of
injury and assist in treatment plan.
5.9.5.5 Non-operative
Treatment Procedures (Sternoclavicular Dislocation /Fracture)
5.9.5.5.1 Symptomatic
posterior dislocations should be reduced in the operating room under general
anesthesia.
5.9.5.5.2 Immobilize
with a sling for 3-4 weeks. Subsequently, further rehabilitation may be
utilized using procedures set forth in Section 5.3.5. Non-operative Treatment
Procedures.
5.9.5.5.3 Medications,
such as analgesics and nonsteroidal anti-inflammatories, would be indicated;
narcotics may be indicated acutely for fracture and should be prescribed as
indicated use is indicated in this Section 6.5 Medications.
5.9.5.6 Operative
Procedures (Sternoclavicular Dislocation/Fracture) would be warranted following
failure of reduction by manipulation with pointed reduction forceps. Caution
should be utilized when pins or screws are used for stabilization secondary to
migration.
5.9.5.7 Post-Operative
Procedures (Sternoclavicular Dislocation/Fracture) would include an
individualized rehabilitation program. This program would begin with 4-6 weeks
of rest with a shoulder immobilizer and be followed by pendulum exercises with
progression to assisted forward flexion and external rotation. Strengthening
exercises should be started at 8-10 weeks.
5.10 SHOULDER
INSTABILITY Subluxation (partial dislocation) or dislocation of the
glenohumeral joint in either an anterior, interior, posterior or
multidirectional position.
5.10.1 History
and Initial Diagnostic Procedures (Shoulder Instability):
5.10.1.1 Occupational
Relationship - instability should be apparent following a direct traumatic blow
to the shoulder, or indirectly by falling on an outstretched arm, or while
applying significant traction to the arm, or may also develop with a cumulative
trauma to the shoulder. Symptoms should be exacerbated or provoked by work and
initially alleviated with a period of rest. Symptoms may be exacerbated by
other activities that are not necessarily work related (e.g., driving a car).
5.10.1.2 History
may include:
5.10.1.2.1 A
slipping sensation in the arm;
5.10.1.2.2 Severe
pain with inability to move the arm;
5.10.1.2.3 Abduction
and external rotation produce a feeling that the shoulder might "come
out"; or
5.10.1.2.4 Feeling
of shoulder weakness.
5.10.1.3 In
subacute and/or chronic instabilities, age of onset of instability is important
in the history. Older age group (over age 40) has a propensity not to
re-dislocate. Younger age groups (under age 30) need a more aggressive treatment
plan.
5.10.1.4 Avoid
any aggressive treatment in patients with history of voluntary subluxation or
dislocation. These patients may need a psychiatric evaluation.
5.10.2 Physical
Findings (Shoulder Instability) may include:
5.10.2.1 Anterior
dislocations would likely include loss of normal shoulder contour; a fullness
in the axilla; pain over the shoulder with any motion and often the patient
holding the extremity in a very still position;
5.10.2.2 Posterior
dislocations usually occur with a direct fall on the shoulder or outstretched
arm resulting in posteriorly directed forces to the humeral head. These
patients present with inability to externally rotate the shoulder;
5.10.2.3 Neurologic
examination could reveal most commonly axillary nerve injuries, but
occasionally musculocutaneous nerve injuries are seen; and/or
5.10.2.4 Abduction
and external rotation positioning will produce pain in those who have anterior
instability. Direct posterior stress in a supine position will produce pain in
those with posterior instability. Longitudinal traction will produce a
"sulcus sign" (a large dimple on the lateral side of the shoulder)
when there is inferior instability.
5.10.3 Laboratory
Tests (Shoulder Instability): are not indicated unless a systemic illness
or disease is suspected.
5.10.4 Testing
Procedures (Shoulder Instability):
5.10.4.1 Plain
x-rays to rule out bony deficit on the glenoid, including AP, axillary view,
lateral in the plane of the scapula and possibly the West
Point view. Axillary view to identify larger Hill-Sachs lesion of
humeral head.
5.10.4.2 On
more difficult diagnostic cases with subtle history and physical findings
suggesting instability, MRI, or a CT assisted arthrogram or MRI assisted
arthrogram may be ordered for lateral detachment after 4-8 weeks of therapy.
(This is done only after other conservative therapies have failed.)
5.10.4.3 An
MRI is indicated to rule out acute rotator cuff injury after shoulder
dislocation in patients over age 45.
5.10.5 Non-operative Treatment Procedures (Shoulder Instability):
5.10.5.1 First-Time
Acute Involvement:
5.10.5.1.1 Therapeutic
Procedures
5.10.5.1.1.1 Immobilization
5.10.5.1.1.2 Therapeutic Exercise
5.10.5.1.1.3 Alteration of Occupation &
Work Station
5.10.5.1.1.4 Thermal Treatment
5.10.5.1.1.5 TENS Unit
5.10.5.1.1.6 Ultrasound
5.10.5.1.2 May
not return to work with overhead activity or lifting with involved arm until
cleared by physician for heavier activities.
5.10.5.1.3 Additional
modalities may include:
5.10.5.1.3.1 Biofeedback
5.10.5.1.3.2 Physical Medicine and
Rehabilitation
5.10.5.1.3.2.1 Instruction in Therapeutic
Exercise and Proper Work Techniques
5.10.5.1.3.2.2 Manual Therapy Techniques
5.10.5.1.3.2.3 Work Conditioning
5.10.5.1.3.2.3.1 Vocational
Rehabilitation
5.10.5.1.3.2.3.2 Vocational Assessment
5.10.5.1.3.2.3.3 Interdisciplinary Team
Approach
5.10.5.1.3.2.3.3.1 Work Hardening
5.10.5.1.3.2.3.3.2 Functional Restoration
Programs
5.10.5.1.3.2.3.3.3 Pain Clinics
5.10.5.1.4 Medications
- medication discussions are in Section 6.5 Medications
5.10.5.1.4.1 Analgesics
5.10.5.1.4.2 Anti-inflammatories
5.10.5.2 Acute or chronic dislocations with large fracture fragments
contributing to instability;
5.10.5.2.1 Attempt
to treat with immobilization if in acceptable position, otherwise repair
surgically
5.10.5.2.2 Return-to-work
may be directly related to time it takes for the fracture to heal
5.10.5.3 Subacute
and/or chronic instability:
5.10.5.3.1 Provocative
dislocation should first be treated similarly to acute dislocation.
5.10.5.3.2 If
acute treatment is unsuccessful, and still having findings of instability,
would consider operative repair.
5.10.6 Operative
Procedures (Shoulder Instability):
5.10.6.1 Identify
causative agent for the instability (i.e., labral detachment, bony lesion, or
multidirectional instability), then proceed with:
5.10.6.1.1 Bony
block transfer;
5.10.6.1.2 Capsular
tightening; or
5.10.6.1.3 Bankart
lesion repair.
5.10.7 Post-Operative
Procedures (Shoulder Instability): would include an individualized rehabilitation
program. Depending upon the type of surgery, the patient will be immobilized
for 3-6 weeks. As soon as it is safe to proceed without damaging the repair,
progressive therapy, either home based or with consultation involving an
occupational and/or physical therapist should begin with therapeutic exercise,
physical medicine and rehabilitation (refer to Section 5.3.5. Non-operative
Treatment Procedures). During this period of time, the patient could resume
working when:
5.10.7.1 A
job assessment results in the treating physician's identification of needed
modifications and restrictions;
5.10.7.2 The
patient has attained a general level of comfort;
5.10.7.3 Medications
which would predispose to injury are no longer being prescribed or used; and
5.10.7.4 The
treating physician has cleared the patient for the specific vocational
activities.
MMI can be expected 6-9 months after
operative intervention. Further job assessment and adjusted work restrictions
may be needed prior to the patients return to full duty.
Before initiation of any therapeutic
procedure, the authorized treating provider, employer and insurer
must
consider these important issues in the care of the injured worker.
First, patients undergoing therapeutic procedure(s) should be released or
returned to modified or
restricted duty during their rehabilitation at the earliest appropriate time.
Refer to “Return-to-Work” in
this section for detailed information.
Second, cessation and/or review of treatment
modalities should be undertaken when no further significant subjective or
objective improvement in the patient’s condition is noted. If patients are not
responding within the recommended duration periods, alternative treatment
interventions, further diagnostic studies or consultations should be pursued.
Third, providers should provide and document
education to the patient. No treatment plan is complete without addressing
issues of individual and/or group patient education as a means of facilitating
self-management of symptoms.
In cases where a patient is unable to attend
an outpatient center, home therapy may be necessary. Home therapy may include
active and passive therapeutic procedures as well as other modalities to assist
in alleviating pain, swelling, and abnormal muscle tone. Home therapy is
usually of short duration and continues until the patient is able to tolerate
coming to an outpatient center.
The following procedures are listed in
alphabetical order.
6.1 ACUPUNCTURE
is an accepted and widely used procedure for the relief of pain and inflammation. The exact mode of action is only partially understood. Western medicine studies suggest that acupuncture stimulates the nervous system at the level of the brain, promotes deep relaxation, and affects the release of neurotransmitters. Acupuncture is commonly used as an alternative or in addition to traditional Western pharmaceuticals. While it is commonly used when pain medication is reduced or not tolerated, it may be used as an adjunct to physical rehabilitation and/or surgical intervention to hasten the return of functional activity. Acupuncture should be performed by MD, DO, DC with appropriate training; or a licensed acupuncturist.
6.1.1 Acupuncture:
is the insertion and removal of filiform needles to stimulate acupoints
(acupuncture points). Needles may be inserted, manipulated and retained for a
period of time. Acupuncture can be used to reduce pain and inflammation, and to
increase blood flow to an area and increase range of motion. Indications
include joint pain, joint stiffness, soft tissue pain and inflammation,
paresthesia, post-surgical pain relief, muscle spasm, and scar tissue pain.
·
Time to produce
effect: 3 to 6 treatments
·
Frequency: 1 to 3
times per week
·
Course duration:
14 treatments
6.1.2 Acupuncture
with Electrical Stimulation: is the use of electrical current (micro- amperage
or milliamperage) on the needles at the acupuncture site. It is used to
increase effectiveness of the needles by continuous stimulation of the
acupoint. Physiological effects (depending on location and settings) can
include endorphin release for pain relief, reduction of inflammation, increased
blood
circulation, analgesia through interruption of pain stimulus, and muscle
relaxation. It is indicated to treat chronic pain conditions, radiating pain
along a nerve pathway, muscle spasm, inflammation, scar tissue pain, and pain
located in multiple sites.
·
Time to produce
effect: 3 to 6 treatments
·
Frequency: 1 to 3
times per week
·
Course duration:
14 treatments
6.1.3 Other
Acupuncture Modalities: Acupuncture treatment is based on individual
patient needs and therefore treatment may include a combination of procedures
to enhance treatment effect. Other procedures may include the use of heat, soft
tissue manipulation/massage, and exercise.
·
Time to produce
effect: 3 to 6 treatments
·
Frequency: 1 to 3
times per week
• Course duration: 14 treatments Any of the
above acupuncture treatments may extend longer if objective functional gains
can be documented or when symptomatic benefits facilitate progression in the
patient’s treatment
program.
Treatment beyond 14 sessions (1 course) may be documented with respect to need
and ability to facilitate positive symptomatic or functional gains.
6.2 BIOFEEDBACK is a
form of behavioral medicine that helps patients learn self-awareness and
self-regulation skills for the purpose of gaining greater control of their
physiology, such as muscle activity, brain waves, and measures of autonomic
nervous system activity. Electronic instrumentation is used to monitor the targeted
physiology and then displayed or fed back to the patient visually,
auditorially, or tactilely, with coaching by a biofeedback specialist.
Biofeedback is provided by clinicians certified in biofeedback and/or who have
documented specialized education, advanced training, or direct or supervised
experience qualifying them to provide the specialized treatment needed (e.g.,
surface EMG, EEG, or other).
Treatment
is individualized to the patient’s work-related diagnosis and needs. Home
practice of skills is required for mastery and may be facilitated by the use of
home training tapes. The ultimate goal in biofeedback treatment is normalizing
the physiology to the pre-injury status to the extent possible and involves
transfer of learned skills to the workplace and daily life. Candidates for
biofeedback therapy or training must be motivated to learn and practice
biofeedback and self-regulation techniques.
Indications
for biofeedback include individuals who are suffering from musculoskeletal
injury where muscle dysfunction or other physiological indicators of excessive
or prolonged stress response affects and/or delays recovery. Other applications
include training to improve self-management of emotional stress/pain responses
such as anxiety, depression, anger, sleep disturbance, and other central and
autonomic nervous system imbalances. Biofeedback is often utilized along with
other treatment modalities.
·
Time to produce
effect: 3 to 4 sessions
·
Frequency: 1 to 2
times per week
·
Maximum duration:
10 to 12 sessions. Treatment beyond 12 sessions must be documented with respect
to need, expectation, and ability to facilitate positive symptomatic or
functional gains.
6.3 INJECTIONS –
THERAPEUTIC are generally accepted, well-established procedures that may
play a significant role in the treatment of patients with upper extremity pain
or pathology. Therapeutic injections involve the delivery of anesthetic and/or
anti-inflammatory medications to the painful structure. Therapeutic injections
have many potential benefits. Ideally, a therapeutic injection will: (a) reduce
inflammation in a specific target area; (b) relieve secondary muscle spasm; and
(c) diminish pain and support therapy directed to functional recovery.
Diagnostic and therapeutic injections should be used early and selectively to
establish a diagnosis and support rehabilitation. If injections are overused or
used outside the context of a monitored rehabilitation program, they may be of
significantly less value.
6.3.1 Steroid
Injections: may provide both diagnostic and therapeutic value in treating a
variety of shoulder disorders. These include biceps tendonitis, bursitis,
rotator cuff tendonitis and impingement syndrome.
Steroid
injections provide a potent anti-inflammatory effect, which is usually short
term in duration,
lasting weeks or months. Injections should always be used as an adjunctive
treatment in the
context of a physical exercise and rehabilitation program.
***When
performing tendon injections, the risk of tendon rupture should be discussed
with the
patient
and the need for temporary restricted duty emphasized. ****
Contraindications: General contraindications include local or systemic
infection, bleeding
disorders, and allergy to medications used.
Local
Steroid Injections:
·
Time to produce
effect: 3 days
·
Frequency:
monthly
·
Maximum duration:
3 injections
6.3.2 Trigger
Point Injections: are generally accepted, although used infrequently in
uncomplicated cases. They may, however, be used to relieve myofascial pain and
facilitate active therapy and stretching of the affected areas, and as an
adjunctive treatment in combination with other treatment modalities, such as
functional restoration programs, including stretching therapeutic exercise.
Trigger point injections should be utilized primarily for the purpose of
facilitating functional progress. The Division does not recommend their routine
use in the treatment of upper extremity injuries.
·
Time to produce
effect: Local anesthetic 30 minutes; 24 to 48 hours for no anesthesia.
·
Frequency:
Weekly. Suggest no more than 4 injection sites per session per week to avoid
significant post-injection soreness.
·
Maximum duration:
8 weeks. Occasional patients may require 2 to 4 repetitions of trigger point
injection series over a 1 to 2 year period.
6.4 JOB SITE ALTERATION
Early evaluation and training of body mechanics and other ergonomic factors are
essential for every injured worker and should be done by a qualified
individual. In some cases, this requires a job site evaluation. Some evidence
supports alteration of the work site in the early treatment of non-traumatic
Shoulder Disorders. There is no single factor or combination of factors that is
proven to prevent or ameliorate Shoulder Disorders, but a combination of
ergonomic are generally considered to be important. Physical factors that may
be considered include use of force, repetition, awkward positions, upper
extremity vibration, cold environment, and contact pressure on the nerve.
The
job analysis and modification should include input from the employee, employer,
and ergonomist or other professional familiar with work place evaluation. The
employee must be observed performing all job functions in order for the job
site analysis to be valid. Periodic follow-up is recommended to evaluate
effectiveness of the intervention and need for additional ergonomic changes.
6.5 MEDICATIONS For
shoulder disorders, medications play a secondary role and should never be the
sole modality of treatment. If a patient's symptoms resolve quickly with
medications or any other passive modality, the practitioner should still
consider prescribing a brief course in shoulder and upper extremity education
and safety. When required, a wide range of medication is available. Modalities
in this group are generally accepted, established and widely used. All
narcotics and habituating medications should be
prescribed
with strict time, quantity and duration guidelines with a definite cessation
parameter. Prescribing these drugs on an as-needed basis (PRN) should almost
always be avoided.
6.5.1 NONSTEROIDAL
ANTI-INFLAMMATORY DRUGS (NSAIDs) are probably the most useful medications
in acute and chronic shoulder injury. In mild cases, they may be the only drug
required for analgesia. There are several classes of NSAIDs and the response of
the individual patient to a specific medication is unpredictable. For this
reason, a range of anti-inflammatory medications may be tried in each case with
the most effective preparation being continued.
For prolonged use of NSAIDs greater than 1-3
months, patients should be monitored for adverse reactions. Appropriate
intervals for metabolic screening are dependent upon the patient's age, general
health status and should be within parameters listed for each specific
medication.
6.5.2 ANALGESICS
(acetaminophen and aspirin are the common choice for non-narcotic analgesia.
6.5.3 PSYCHOTROPIC
MEDICATION may be used in patients with a high level of anxiety or
depression. A variety of psychotropic drugs may be used. In acute or subacute
shoulder injury, these medications are generally unnecessary except for the use
of tricyclic antidepressants as substitutes for hypnotics and/or analgesics. In
most cases, major tranquilizers, anxiolytics and antidepressants are reserved
for chronic pain disorders. Patients, whose chief complaint is shoulder injury,
but require use of major tranquilizers or anxiolytics for greater than two
weeks. In particular, benzodiazepams are almost always contraindicated in
patients with shoulder injury unless a severe anxiety state exist requiring
psychiatric supervision or in cases of extremely severe, objectively visualized
acute muscle spasm. In this type of acute scenario, the maximum duration for benzodiazepam
administration should be limited to less than five days.
6.5.4 HYPNOTICS
may be given to shoulder injury sufferers because of a chief complaint of
"inability to sleep." Such medication must be used with caution
because of their dependence-producing capabilities. The Division recommends
consideration of sedating tricyclic antidepressants as an alternative when
necessary. Physical methods of restoring a normal sleep pattern can usually be
employed as an alternative to medication.
6.5.5 NARCOTICS
should be primarily reserved for the treatment of acute shoulder injury or
the treatment of patients with objectively documented acute exacerbations. The
action of these drugs is central, affecting the patient's perception of pain
rather than the pain process itself.
Narcotics are rarely indicated in the
treatment of patients with pure shoulder injury without fracture. In mild to
moderate cases of upper extremity pain, narcotic medication should not be used
at all. Adverse effects include respiratory depression and the development of
physical and psychological dependence.
6.5.6 MINOR
TRANQUILIZERS/MUSCLE RELAXANTS should be primarily reserved for the
treatment of acute shoulder with muscle spasm or the treatment of patients with
objectively documented acute exacerbations. Muscle relaxants may have a
significant effect on the early phases of acute shoulder disorders. Their
action is central and with no effect on the neuromuscular junction of the
muscles themselves. Purported peripheral effects are difficult to separate from
the anxiolytic central action.
6.6 OCCUPATIONAL
REHABILITATION PROGRAMS
6.6.1 Non-Interdisciplinary: These programs are
work-related, outcome-focused, individualized treatment programs. Objectives of
the program include, but are not limited to, improvement of cardiopulmonary and
neuromusculoskeletal functions (strength, endurance, movement, flexibility,
stability, and motor control functions), patient education, and symptom relief.
The goal is for patients to gain full or optimal function and return-to-work.
The service may include the time-limited use of passive modalities with
progression to achieve treatment and/or simulated/real work.
6.6.1.1 WORK CONDITIONING/SIMULATION
This program may begin once a patient is out
of the acute phase of injury and will be able
to tolerate this program.
These programs are usually initiated after
the acute phase has been completed and
offered at any time throughout the recovery
phase. Work conditioning should be initiated when imminent return of a patient
to modified or full duty is not an option, but the prognosis for returning the
patient to work at completion of the program is at least fair to good.
The need for work place simulation should be
based upon the results of a Functional Capacity Evaluation and/or Jobsite
Analysis.
·
Length of visit:
1 to 4 hours per day.
·
Frequency: 2 to 5
visits per week
·
Maximum Duration:
8 WEEKS. Participation in a program beyond six weeks must be documented with
respect to need and the ability to facilitate positive symptomatic or
functional gains.
6.6.2 WORK
HARDENING Work Hardening is an interdisciplinary program addressing a
patient’s employability and return to work. It includes a progressive increase
in the number of hours per day that a patient completes work simulation tasks
until the patient can tolerate a full workday. This is accomplished by
addressing
the medical, behavioral, physical, functional, and vocational components of
employability and return-to-work. This can include a highly structured program
involving a team approach or can involve any of the
components thereof. The interdisciplinary
team should, at a minimum, be comprised of a qualified medical director who is
board certified with documented training in occupational rehabilitation; team
physicians having experience in occupational rehabilitation; occupational
therapist; physical therapist; case manager; and psychologist. As appropriate,
the team may also include: chiropractor, RN, vocational specialist or Certified
Biofeedback Therapist.
·
Length of visit:
up to 8 hours/day
·
Frequency: 2 to 5
visits per week
·
Maximum duration:
8 weeks. Participation in a program beyond six weeks must be documented with
respect to need and the ability to facilitate positive symptomatic or
functional gains.
6.7 PATIENT
EDUCATION No treatment plan is complete without addressing issues of
individual patient and/or group education as a means of prolonging the
beneficial effects of treatment, as well as facilitating self-management of
symptoms and injury prevention. The patient should take an active role in the
establishment of functional outcome goals, and should be educated on his or her
specific injury, assessment findings, and plan of treatment. Education and
instruction in proper body mechanics and posture, positions to avoid task/tool
adaptation, self-care for exacerbation of symptoms, and home exercise/task
adaptation should also be addressed.
·
Time to produce
effect: Varies with individual patient.
·
Frequency: Should
occur at every visit.
6.8 RETURN-TO-WORK
is therapeutic, assuming the work is not likely to aggravate the basic
problem or increase long-term pain. The practitioner must provide specific
physical limitations per the Physician’s Form. The following physical
limitations should be considered and modified as recommended: lifting, pushing,
pulling, crouching, walking, using stairs, bending at the waist, awkward and/or
sustained postures, tolerance for sitting or standing, hot and cold
environments, data entry and other repetitive motion tasks, sustained grip,
tool usage and vibration factors. Even if there is residual chronic pain,
return-to-work is not necessarily contraindicated.
The practitioner should understand all of
the physical demands of the patient’s job position before returning the patient
to full duty and should receive clarification of the patient’s job duties.
6.9 SLEEP
DISTURBANCES are a common secondary symptom of CTD. Although primary
insomnia may accompany pain as an independent co-morbid condition, it more
commonly occurs, secondary to the pain condition itself. Exacerbations of pain
often are accompanied by exacerbations of insomnia; the reverse can also occur.
Sleep laboratory studies have shown disturbances of sleep architecture in pain
patients. Loss of deep slow-wave sleep and increase in light sleep occur and
sleep efficiency, the proportion of time in bed spent asleep, is decreased. These
changes are associated with patient reports of non-restorative sleep.
Many affected patients develop behavioral
habits that exacerbate and maintain sleep disturbances. Excessive time in bed,
irregular sleep routine, napping, low activity, and worrying in bed are all
maladaptive responses that can arise in the absence of any psychopathology.
There is some evidence that behavioral modification, such as patient education
and group or individual counseling, can be effective in reversing the effects
of insomnia. Behavioral modifications are easily implemented and can include:
6.9.1 Maintaining
a regular sleep schedule, retiring and rising at approximately the same time on
weekdays and weekends.
6.9.2 Avoiding
daytime napping.
6.9.3 Avoiding
caffeinated beverages after lunchtime
6.9.4 Making
the bedroom quiet and comfortable, eliminating disruptive lights, sounds,
television sets, and keeping a bedroom temperature of about 65°F.
6.9.5 Avoiding
alcohol or nicotine within two hours of bedtime.
6.9.6 Avoiding
large meals within two hours of bedtime.
6.9.7 Exercising
vigorously during the day, but not within two hours of bedtime, since this may
raise core temperature and activate the nervous system.
6.9.8 Associating
the bed with sleep and sexual activity only, using other parts of the home for
television, reading and talking on the telephone.
6.9.9 Leaving
the bedroom when unable to sleep for more than 20 minutes, retuning to the
bedroom
when
ready to sleep again.
These modifications should be undertaken before sleeping medication is
prescribed for long term
use.
6.10 THERAPY–PASSIVE
includes those treatment modalities that do not require energy expenditure
on the part of the patient. They are principally effective during the early
phases of treatment and are directed at controlling symptoms such as pain,
inflammation and swelling and to improve the rate of healing soft tissue
injuries. They should be used in adjunct with active therapies to help control
swelling, pain and inflammation during the rehabilitation process. They may be
used intermittently as a therapist deems appropriate or regularly if there are
specific goals with objectively measured functional improvements during
treatment.
6.10.1 Electrical
Stimulation (Unattended and Attended): once applied, requires minimal
on-site supervision by the physician or non-physician provider. Indications
include pain, inflammation, muscle spasm, atrophy, and decreased circulation.
·
Time to produce
effect: 2 to 4 treatments
·
Frequency:
Varies, depending upon indication, between 2 to 3 times/day to 1 time/week.
Provide home unit if frequent use.
·
Maximum duration:
24 visits
6.10.2 Extracorporeal
shock wave treatment: Consists of the application of pulses of high
pressure sound to soft tissues, similar to lithotriptors. It has been
investigated for its effectiveness in the treatment of Calcific Tendonitis. It
has not been shown to have an advantage over other conservative treatments and
remains investigational. It is not recommended.
6.10.3 Iontophoresis:
is the transfer of medication, including, but not limited to, steroidal
antiinflammatories and anesthetics, through the use of electrical stimulation.
Indications include pain (Lidocaine), inflammation (hydrocortisone,
salicylate), edema (mecholyl, hyaluronidase, salicylate), ischemia (magnesium,
mecholyl, iodine), muscle spasm (magnesium, calcium), calcific deposits
(acetate), scars and keloids (chlorine, iodine, acetate).
·
Time to produce
effect: 1 to 4 treatments
·
Frequency: 3
times per week with at least 48 hours between treatments.
·
Maximum duration:
8 treatments per region
6.10.4 Laser
irradiation: Consists of the external application of an array of visible
and infrared wavelengths to soft tissues. Frequency and duration are dependent
on severity and chronicity of problem.
6.10.5 Manual
Therapy Techniques: are passive interventions in which the providers use
his or her hands to administer skilled movements designed to modulate pain;
increase joint range of motion; reduce/eliminate soft tissue swelling,
inflammation, or restriction; induce relaxation; and improve contractile and
non-contractile tissue extensibility. These techniques are applied only after a
thorough examination is performed to identify those for whom manual therapy
would be contraindicated or for whom manual therapy must be applied with
caution.
6.10.5.1 MANIPULATION:
is generally accepted, well-established and widely used therapeutic
intervention for low back pain. Manipulative Treatment (not therapy) is defined
as the therapeutic application of manually guided forces by an operator to
improve physiologic function and/or support homeostasis that has been altered
by the injury or occupational disease, and has associated clinical
significance.
High
velocity, low amplitude (HVLA) technique, chiropractic manipulation,
osteopathic manipulation, muscle energy techniques, counter strain, and
non-force techniques are all types of manipulative treatment. This may be
applied by osteopathic physicians (D.O.), chiropractors (D.C.), properly
trained physical therapists (P.T.), or properly trained medical physicians.
Under these different types of manipulation exist many subsets of different
techniques that can be described as a) direct- a forceful engagement of a
restrictive/ pathologic barrier, b) indirect- a gentle/non-forceful
disengagement of a restrictive/ pathologic barrier, c) the patient actively
assists in the treatment and d) the patient relaxing, allowing the practitioner
to move the body tissues. When the proper diagnosis is made and coupled with
the appropriate technique, manipulation has no contraindications and can be
applied to all tissues of the body. Pre-treatment assessment should be
performed as part of each manipulative treatment visit to ensure that the
correct diagnosis and correct treatment is employed.
High
velocity, low amplitude (HVLA) manipulation is performed by taking a joint to
its end range of motion and moving the articulation into the zone of accessory
joint movement, well within the limits of anatomical integrity. Indications for
manipulation include joint pain, decreased joint motion, and joint adhesions.
Contraindications to HVLA manipulation include joint instability, fractures,
severe osteoporosis, infection, metastatic cancer, active inflammatory
arthritides, aortic aneurysm, and signs of progressive neurologic deficits.
Time
to produce effect for all types of manipulative treatment: 1 to 6 treatments.
·
Frequency: Up to
3 times per week for the first 4 weeks as indicated by the severity of
involvement and the desired effect, then up to 2 treatments per week for the
next 4 weeks. For further treatments, twice per week or less to maintain
function.
·
Maximum duration:
48 visits. Extended durations of care beyond what is considered “maximum” may
be necessary in cases of re-injury, interrupted continuity of care,
exacerbation of symptoms, and in those patients with comorbidities. Refer to
the Chronic Pain Guidelines for care beyond 6 months.
The
combination of 97140 plus either CMT or OMT code is equal to one visit when
performed on the same day. Any combination of manual therapeutic intervention
exceeding 48 visits (not units) needs to go to UR.
6.10.5.2 MOBILIZATION
(Joint) /Manipulation Mobilization is passive movement involving
oscillatory motions to the involved joints. The passive mobility is performed
in a graded manner (I, II, III, IV, or V), which depicts the speed of the
maneuver. It may include skilled manual joint tissue stretching. Indications
include
the need to improve joint play, improve intracapsular arthrokinematics, or
reduce pain associated with tissue impingement.
·
Time to produce
effect: 4 to 6 treatments
·
Frequency: 2 to 3
times per week
·
Maximum duration:
48 visits (CPT codes 97124 and 97140 can not exceed 48 visits in combination).
6.10.5.3 MOBILIZATION
(Soft Tissue) Mobilization of soft tissue is the skilled application of
manual techniques designed to normalize movement patterns through the reduction
of soft tissue pain and restrictions.
Indications
include muscle spasm around a joint, trigger points, adhesions, and neural
compression.
Nerve Gliding: consist of a series of flexion and
extension movements of the hand, wrist, elbow, shoulder, and neck that produce
tension and longitudinal movement along the length of the median and other
nerves of the upper extremity. These exercises are based on the principle that
the tissues of the peripheral nervous system are designed for movement, and
that tension and glide (excursion) of nerves may have an effect on
neurophysiology through alterations in vascular and axoplasmic flow.
Biomechanical principles have been more thoroughly studied than clinical
outcomes. Nerve gliding performed on a patient by the clinician should be
reinforced by patient performance of similar techniques as part of a home
exercise program at least twice per day.
·
Time to produce
effect: 4 to 6 treatments
·
Frequency: 2 to 3
times per week
·
Maximum duration:
48 visits (CPT codes 97124 and 97140 can not exceed 48 visits in combination).
6.10.6 Massage:
Manual or Mechanical - Massage is manipulation of soft tissue with broad
ranging relaxation and circulatory benefits. This may include stimulation of
acupuncture points and acupuncture channels (acupressure), application of
suction cups and techniques that include pressing, lifting, rubbing, pinching
of soft tissues by or with the practitioner’s hands. Indications include edema,
muscle spasm, adhesions, the need to improve peripheral circulation and range
of motion, or to increase muscle relaxation and flexibility prior to exercise.
·
Time to produce
effect: Immediate.
·
Frequency: 1 to 3
times per week
·
Maximum duration:
12 visits (CPT codes 97124 and 97140 can not exceed 48 visits in combination).
6.10.7 Orthotics/Immobilization
with Splinting: is a generally accepted, well-established and widely used
therapeutic procedure. Splints may be effective when worn at night or during
portions of the day, depending on activities. Splints should be loose and soft
enough to maintain comfort while supporting the involved joint in a relatively
neutral position. Splint comfort is critical and may affect compliance.
Although off-the-shelf splints are usually sufficient, custom thermoplastic
splints may provide better fit for certain patients.
Splints may be effective when worn at night
or during portions of the day, depending on activities; however, splint use is
rarely mandatory. Providers should be aware that over-usage is counterproductive,
and counsel patients to minimize daytime splint use in order avoid detrimental
effects, such as, stiffness and dependency over time.
·
Time to produce
effect: 1-4 weeks
·
Frequency:
Daytime intermittent or night use, depending on symptoms and activities.
·
Maximum duration:
2 to 4 months. If symptoms persist, consideration should be given to further
diagnostic studies or to other treatment options.
6.10.8 Superficial
Heat and Cold Therapy: are thermal agents applied in various manners that
lowers or raises the body tissue temperature for the reduction of pain,
inflammation, and/or effusion resulting from injury or induced by exercise.
Includes application of heat just above the surface of the skin at acupuncture
points. Indications include acute pain, edema and hemorrhage, need to increase
pain threshold, reduce muscle spasm and promote stretching/flexibility. Cold
and heat packs can be used at home as an extension of therapy in the clinic
setting.
·
Time to produce
effect: Immediate
·
Frequency: 2 to 5
times per week (clinic). Home treatment as needed.
·
Maximum duration:
12 visits, with maximum vists 1 per day. If symptoms persist, consideration should be given to further
diagnostic studies or other treatment options.
6.10.9 Ultrasound:
uses sonic generators to deliver acoustic energy for therapeutic thermal
and/or nonthermal soft tissue effects. Indications include scar tissue,
adhesions, collagen fiber and muscle spasm, and to improve muscle tissue extensibility
and soft tissue healing. Ultrasound with electrical stimulation is concurrent
delivery of electrical energy that involves dispersive electrode placement.
Indications include muscle spasm, scar tissue, pain modulation and muscle
facilitation.
Phonophoresis is the transfer of medication
to the target tissue to control inflammation and pain through the use of sonic
generators. These topical medications include, but are not limited to,
steroidal anti-inflammatory and anesthetics.
·
Time to produce
effect: 4 to 8 treatments
·
Frequency: 2-3
times per week
·
Maximum duration:
18 visits
6.11 THERAPY–ACTIVE
therapies are based on the philosophy that therapeutic exercise and/or
activity are beneficial for restoring flexibility, strength, endurance,
function, range of motion, and alleviating discomfort. Active therapy requires
an internal effort by the individual to complete a specific exercise or task,
and thus assists in developing skills promoting independence to allow self-care
after discharge. This form of therapy requires supervision from a therapist or
medical provider such as verbal, visual, and/or tactile instructions. At times
a provider may help stabilize the patient or guide the movement pattern but the
energy required to complete the task is predominately executed by the patient.
Patients should be instructed to continue
active therapies at home as an extension of the treatment process in order to
maintain improvement levels. Home exercise can include exercise with or without
mechanical assistance or resistance and functional activities with assistive
devices.
Interventions are selected based on the
complexity of the presenting dysfunction with ongoing examination, evaluation
and modification of the plan of care as improvement or lack thereof occurs.
Change and/or discontinuation of an intervention should occur if there is
attainment of expected goals/ outcome, lack of progress, lack of tolerance
and/or lack of motivation. Passive interventions/modalities may only be used as
adjuncts to the active program.
6.11.1 Activities
of Daily Living: Supervised instruction, active-assisted training, and/or
adaptation of activities or equipment to improve a person’s capacity in normal
daily living activities such as self-care, work re-integration training,
homemaking, and driving.
·
Time to produce
effect: 4 to 5 treatments
·
Maximum of 10
sessions
6.11.2 Aquatic
Therapy: is a well-accepted treatment which consists of the therapeutic use
of aquatic immersion for therapeutic exercise to promote strengthening, core
stabilization, endurance, range of motion, flexibility, body mechanics, and
pain management. Aquatic therapy includes the implementation of active
therapeutic procedures in a swimming or therapeutic pool. The water provides a
buoyancy force that lessens the amount of force gravity applies to the body.
The decreased gravity effect allows the patient to have a mechanical advantage
and more likely have a successful trial of therapeutic exercise. The therapy
may be indicated for individuals who:
·
cannot tolerate
active land-based or full-weight bearing therapeutic procedures
·
require increased
support in the presence of proprioceptive deficit;
·
are at risk of
compression fracture due to decreased bone density;
·
have symptoms
that are exacerbated in a dry environment;
·
would have a
higher probability of meeting active therapeutic goals than in a land-
·
based
environment. The pool should be large enough to allow full extremity range of
motion and fully erect posture. Aquatic vests, belts and other devices can be
used to provide stability, balance, buoyancy, and resistance.
·
Time to produce
effect: 4 to 5 treatments
·
Frequency: 3 to 5
times per week
·
Maximum duration:
24 visits
A self-directed program is recommended after
the supervised aquatics program has been established, or, alternatively a
transition to a land-based environment exercise program.
6.11.3 Functional
Activities: are the use of therapeutic activity to enhance mobility, body mechanics,
employability, coordination, and sensory motor integration.
·
Time to produce
effect: 4 to 5 treatments
·
Frequency: 3 to 5
times per week
·
Maximum duration:
24 visits
Total number of visit 97110 and 97530 should
not exceed 36 visits without pre-authorization
6.11.4 Neuromuscular
Re-education: is the skilled application of exercise with manual,
mechanical, or electrical facilitation to enhance strength, movement patterns,
neuromuscular response, proprioception, kinesthetic sense, coordination
education of movement, balance, and posture. Indications include the need to
promote neuromuscular responses through carefully timed proprioceptive stimuli,
to elicit and improve motor activity in patterns similar to normal
neurologically developed sequences, and improve neuromotor response with
independent control.
·
Time to produce
effect: 2 to 6 treatments
·
Frequency: 3-5
times per week
·
Maximum duration:
24 visits
6.11.5 Proper
Work Techniques: Please refer to the “Job Site Evaluation” and “Job Site
Alteration” sections of these guidelines.
6.11.6 Therapeutic
Exercise: with or without mechanical assistance or resistance may include
isoinertial, isotonic, isometric and isokinetic types of exercises. Indications
include the need for cardiovascular fitness, reduced edema, improved muscle
strength, improved connective tissue strength and integrity, increased bone
density, promotion of circulation to enhance soft tissue healing, improvement
of muscle recruitment, increased range of motion, and are used to promote
normal movement patterns. Can also include complimentary/alternative exercise
movement therapy.
·
Time to produce
effect: 2 to 6 treatments
·
Frequency: 3 to 5
times per week
• Maximum duration: 36 visits Total number of visit
97110 and 97530 should not exceed 36 visits without pre authorization
6.12 RESTRICTION
OF ACTIVITIES Continuation of normal daily activities is the recommendation
for most Shoulder Disorders with or without neurologic symptoms. Complete work
cessation should be avoided, if possible, since it often further aggravates the
pain presentation. Modified return-to-work is almost always more efficacious
and rarely contraindicated in the vast majority of injured workers with
Shoulder Disorders.
6.13 VOCATIONAL
REHABILITATION is a generally accepted intervention. Initiation of
vocational rehabilitation requires adequate evaluation of patients for
quantification of highest functional level, motivation, and achievement of
maximum medical improvement. Vocational rehabilitation may be as simple as
returning to the original job or as complicated as being retrained for a new
occupation.
PART F CERVICAL TREATMENT GUIDELINES
|
Pursuant to 19 Del.C. §2322C, health care
practice guidelines have been adopted and recommended by the Health Care
Advisory Panel to guide utilization of health care treatments in workers'
compensation including, but not limited to, care provided for the treatment of
employees by or under the supervision of a licensed health care provider,
prescription drug utilization, inpatient hospitalization and length of stay,
diagnostic testing, physical therapy, chiropractic care and palliative care.
The health care practice guidelines apply to all treatments provided after the
effective date of the regulation adopted by the Department of Labor, May 23,
2008, and regardless of the date of injury. The cervical treatment guidelines were added to the list of treatment guidelines, effective June 1, 2009. The guidelines are, to the extent
permitted by the most current medical science or applicable science, based on
well-documented scientific research concerning efficacious treatment for
injuries and occupational disease. To the extent that well-documented
scientific research regarding the above is not available at the time of,
adoption of the guidelines, or is not available at the time of any revision to
the guidelines, the guidelines have been and will be based upon the best
available information concerning national consensus regarding
best health care practices in the relevant health care community.
The guidelines, to the extent practical
and consistent with the Act, address treatment of those physical conditions which occur with the
greatest frequency, or which require the most expensive treatments, for work-related injuries based upon
currently available Delaware
data.
Services rendered by any health care
provider certified pursuant to 19 Del.C. §2322D(a) to provide treatment or services for injured
employees shall be presumed, in the absence of contrary evidence, to be reasonable and necessary if such
treatment and/or services conform to the most current version of the Delaware health care practice guidelines.
Services rendered outside the
Guidelines and/or variation in treatment recommendations from the Guidelines
may represent acceptable medical care, be considered reasonable and necessary
treatment and, therefore, determined to be compensable, absent evidence to the
contrary, and may be payable in accordance with the Fee
Schedule and Statute, accordingly.
Services provided by any health care provider that is not certified pursuant to
19 Del.C. §2322D(a)
shall not be presumed reasonable and necessary unless such services are
preauthorized by the
employer or insurance carrier, subject to the exception set forth in 19 Del.C.
§2322D(b).
Treatment of
conditions unrelated to the injuries sustained in an industrial accident may be
denied as unauthorized if the treatment is directed toward the non-industrial
condition, unless the treatment of the unrelated injury is rendered necessary
as a result of the industrial accident.
The Health Care
Advisory Panel and Department of Labor recognized that acceptable medical
practice may include deviations from these Guidelines, as individual cases
dictate. Therefore, these Guidelines are not relevant as evidence of a
provider's legal standard of professional care.
In accordance
with the requirements of the Act, the development of the health care guidelines
has been directed by a predominantly medical or other health professional
panel, with recommendations then made to the Health Care Advisory Panel.
|
|
2.1 TREATMENT PARAMETER With respect
to Therapy (Active or Passive), time frames/visits for specific interventions
commence once treatments have been initiated, not on the date of injury.
Obviously, duration will be impacted by patient compliance, as well as
comorbitities and availability of services. Clinical judgment may substantiate
the need to accelerate or decelerate modify the time frames total number of
visits discussed in this document. The majority of injured workers with
Cervical pain often will achieve resolution of their condition within 8 to 24
visits (Guide to Physical Therapy Practice - Second Edition). It is anticipated
that most injured workers will not require the maximum number of visits
described in these guidelines. They are designed to be a ceiling and care
extending beyond the maximum allowed visits may warrant utilization review.
2.2 ACTIVE INTERVENTIONS emphasizing
patient responsibility, such as therapeutic exercise and/or functional
treatment, are generally emphasized over passive modalities, especially as
treatment progresses. Generally, passive interventions are viewed as a means to
facilitate progress in an active rehabilitation program with concomitant
attainment of objective functional gains. All rehabilitation programs must
incorporate "Active Interventions" no later than twelve visits or
three weeks after the onset of treatment. Reimbursement for passive modalities
only after the first twelve visits three weeks of treatment without clear
evidence of Active Interventions will require supportive documentation.
2.3 ACTIVE THERAPEUTIC EXERCISE PROGRAM
goals should incorporate patient strength, endurance, flexibility,
coordination, and education. This includes functional application in vocational
or community settings.
2.4 POSITIVE PATIENT RESPONSE results
are defined primarily as functional gains that can be objectively measured.
Objective functional gains include, but are not limited to, positional
tolerances, range of motion (ROM), strength, endurance, activities of daily
living, cognition, behavior, and efficiency/velocity measures that can be
quantified. Subjective reports of pain and function should be considered and
given relative weight when the pain has anatomic and physiologic correlation.
2.5 RE-EVALUATE TREATMENT EVERY 3 TO 4
WEEKS With respect to Therapy (Active or Passive), if a given treatment or
modality is not producing positive results within 3 to 4 weeks, the treatment
may be either modified or discontinued. Reconsideration of diagnosis should
also occur in the event of poor response to a seemingly rational intervention.
2.6 SURGICAL INTERVENTIONS should be
contemplated within the context of expected functional outcome and not purely
for the purpose of pain relief. All operative interventions must be based upon
positive correlation of clinical findings, clinical course, and diagnostic
tests. A comprehensive assimilation of these factors must lead to a specific
diagnosis with positive identification of pathologic conditions.
2.7 SIX-MONTH TIME FRAME The
prognosis drops precipitously for returning an injured worker to work once
he/she has been temporarily totally disabled for more than six months. The
emphasis within these guidelines is to move patients along a continuum of care
and return to work within a six-month time frame, whenever possible. It is
important to note that time frames may not be pertinent to injuries that do not
involve work-time loss or are not occupationally related.
2.8 RETURN-TO-WORK is therapeutic,
assuming the work is not likely to aggravate the basic problem or increase
long-term pain. The following physical limitations should be considered and
modified as recommended: lifting, pushing, pulling, crouching, walking, using
stairs, bending at the waist, awkward and/or sustained postures, tolerance for
sitting or standing, hot and cold environments, data entry and other repetitive
motion tasks, sustained grip, tool usage and vibration factors. Even if there
is residual chronic pain, return-to-work is not necessarily contraindicated.
The practitioner should understand all of the physical demands of the patient's
job position before returning the patient to full duty and should receive
clarification of the patient's job duties.
2.9 GUIDELINE RECOMMENDATIONS AND
INCLUSION OF MEDICAL EVIDENCE. Recommendations are based on available
evidence and/or consensus recommendations of the standard of care within Delaware. Those
procedures considered inappropriate, unreasonable, or unnecessary are
designated in the guideline as being "not recommended."
2.10 DELAYED RECOVERY. The Department
recognizes that not all industrially injured patients will recover within the
time lines outlined in this document despite optimal care. Such individuals may
require treatments beyond the limits discussed within this document, but such
treatment will require clear documentation by the authorized treating
practitioner focusing on objective functional gains afforded by further
treatment and impact upon prognosis.
|
|
The Division
recommends the following diagnostic procedures be considered, at least
initially, the responsibility of the workers' compensation carrier to ensure
that an accurate diagnosis and treatment plan can be established. Standard
procedures, that should be utilized when initially diagnosing a work-related
Cervical pain complaint, are listed below.
3.1 HISTORY-TAKING AND PHYSICAL
EXAMINATION (Hx & PE) are generally accepted, well established and
widely used procedures that establish the foundation/basis for and dictates
subsequent stages of diagnostic and therapeutic procedures. When findings of
clinical evaluations and those of other diagnostic procedures are not
complementing each other, the objective clinical findings should have
preference. The medical records should reasonably document the following.
3.1.1 History of Present Injury A detailed
history, taken in temporal proximity to the time of injury should primarily
guide evaluation and treatment.
3.1.2 Physical Examination: may include
accepted tests and exam techniques applicable to the area being examined:
3.1.2.1 Visual inspection, including posture;
3.1.2.2 Cervical range-of-motion, quality of
motion, and presence of muscle spasm. Motion evaluation of specific joints may
be indicated. Range-of-motion should not be checked in acute trauma cases until
fracture and instability have been ruled out on clinical examination, with or
without radiographic evaluation;
3.1.2.3 Examination of thoracic spine
3.1.2.4 Palpation of spinous processes, facets,
and muscles noting myofascial tightness, tenderness, and trigger points;
3.1.2.5 Motor and sensory examination of the
upper muscle groups with specific nerve root focus, as well as sensation to
light touch, pin prick, temperature, position and vibration. More than 2 cm
difference in the circumferential measurements of the two upper extremities may
indicate chronic muscle wasting; and
3.1.2.6 Deep tendon reflexes. Asymmetry may
indicate pathology. Inverted reflexes (e.g. arm flexion or triceps tap) may
indicate nerve root or spinal cord pathology at the tested level. Pathologic
reflexes include wrist, clonus, grasp reflex, and Hoffman's sign.
3.1.3 Spinal Cord Evaluation: In cases
where the mechanism of injury, history, or clinical presentation suggests a
possible severe injury, additional evaluation is indicated. A full neurological
examination for possible spinal cord injury may include:
3.1.3.1 Sharp and light touch, deep pressure,
temperature, and proprioceptive sensory function;
3.1.3.2 Strength testing;
3.1.3.3 Anal sphincter tone and/or perianal
sensation;
3.1.3.4 Presence of pathological reflexes of the
upper and lower extremities; or
3.1.3.5 Evidence of an Incomplete Spinal Cord
Injury Syndrome
3.1.3.5.1 Anterior Cord Syndrome is characterized by
the loss of motor function and perception of pain and temperature below the
level of the lesion with preservation of touch, vibration, and proprioception.
This is typically seen after a significant compressive or flexion injury.
Emergent CT or MRI is necessary to look for a possible reversible compressive
lesion requiring immediate surgical intervention. The prognosis for recovery is
the worst of the incomplete syndromes.
3.1.3.5.2 Brown-Sequard Syndrome is characterized by
ipsilateral motor weakness and proprioceptive disturbance with contralateral
alteration in pain and temperature perception below the level of the lesion.
This is usually seen in cases of penetrating trauma or lateral mass fracture.
Surgery is not specifically required, although debridement of the open wound
may be.
3.1.3.5.3 Central Cord Syndrome is characterized by
sensory and motor disturbance of all limbs, often upper extremity more than
lower, and loss of bowel and bladder function with preservation of perianal
sensation. This is typically seen in elderly patients with a rigid spine
following hyperextension injuries. Surgery is not usually required.
3.1.3.5.4 Posterior Cord Syndrome, a rare condition,
is characterized by loss of sensation below the level of the injury, but intact
motor function.
3.1.3.6 Spinal cord lesions may be classified
according to the American Spine Injury Association (ASIA) impairment scale.
|
ASIA IMPAIRMENT SCALE |
A = Complete: No motor or sensory function is
preserved in the sacral segments S4-S5
B = Incomplete: Sensory but not motor function is
preserved below the neurological level and includes
the sacral segments S4-S5
C = Incomplete: Motor function is preserved below
the neurological level, and more than half of key
muscles below the neurological level have a muscle
grade less than 3
D = Incomplete: Motor function is preserved below
the neurological level, and at least half of key
muscles below the neurological level have a grade
of 3 or more
E = Normal: motor and sensory function are normal |
A worksheet which details
dermatomes and muscle testing required is available from ASIA.
3.1.4 Soft Tissue Injury Evaluation: Soft
tissue injuries are traumatic injuries to the muscles, ligaments, tendons,
and/or connective tissue. The most common mechanism is sudden hyperextension
and/or hyperflexion of the neck. Acceleration/deceleration on the lateral plane
may also result in one of these syndromes. A true isolated cervical strain is
not associated with focal neurological symptoms. The signs and pathophysiology
of these injuries are not well understood. Soft tissue injuries may include
cervical strain, myofascial syndromes, somatic dysfunction, and fractures.
3.2 RADIOGRAPHIC IMAGING of the
Cervical spine is a generally accepted, well-established and widely used
diagnostic procedure when specific indications based on history and/or physical
examination are present The mechanism of injury and specific indications for
the radiograph should be listed on the request form to aid the radiologist and
x-ray technician. Suggested indications may include:
3.2.1 History of trauma,
3.2.2 Age over 55 years;
3.2.3 Unexplained or persistent Cervical pain for
at least 6 weeks or pain that is worse with rest;
3.2.4 Localized pain, fever, constitutional
symptoms, or history or exam suggestive of intravenous drug abuse, prolonged
steroid use, or osteomyelitis;
3.2.5 Suspected lesion in the Cervical spine due
to systemic illness such as a rheumatic/rheumatoid disorder or endocrinopathy.
Suspected lesions may require special views;
3.2.6 Past medical history suggestive of
pre-existing spinal disease, osteoporosis, spinal instrumentation, or cancer;
and
3.2.7 Prior to high-velocity/low amplitude
manipulation or Grade IV to V mobilization.
3.3 LABORATORY TESTING Laboratory
tests are generally accepted, well-established and widely used procedures. They
are, however, rarely indicated at the time of initial evaluation, unless there
is suspicion of systemic illness, infection, neoplasia, or underlying
rheumatologic disorder, connective tissue disorder, or based on history and/or
physical examination. Laboratory tests can provide useful diagnostic
information. Tests include, but are not limited to:
3.3.1 Complete blood count (CBC) with
differential can detect infection, blood dyscrasias, and medication side
effects;
3.3.2 Erythrocyte sedimentation rate (ESR),
rheumatoid factor (RF), antinuclear antigen (ANA), human leukocyte antigen
(HLA), and C-reactive protein (CRP), can be used to detect evidence of a
rheumatologic, infectious, or connective tissue disorder;
3.3.3 Serum calcium, phosphorous, uric acid,
alkaline phosphatase, and acid phosphatase can detect metabolic bone disease;
3.3.4 Urinalysis for bacteria (usually with
culture and sensitivity), calcium, phosphorus, hydroxyproline, or hematuria;
and
3.3.5 Liver and kidney
function may be performed for prolonged anti-inflammatory use or other
medications requiring monitoring.
|
|
One diagnostic
imaging procedure may provide the same or distinctive information as does
another procedure. Therefore, the prudent choice of a single diagnostic
procedure, a complement of procedures or a sequence of procedures will optimize
diagnostic accuracy; maximize cost effectiveness (by avoiding redundancy), and
minimize potential adverse effects to patients. All imaging procedures have a
degree of specificity and sensitivity for various diagnoses. No isolated
imaging test can assure a correct diagnosis. Clinical information obtained by
history taking and physical examination should form the basis for selecting an
imaging procedure and interpreting its results. Magnetic resonance imaging
(MRI), myelography, or Computed Axial Tomography (CT) scanning following
myelography may provide useful information for many spinal disorders. When a
diagnostic procedure, in conjunction with clinical information, can provide
sufficient information to establish an accurate diagnosis, the second
diagnostic procedure will become a redundant procedure. At the same time, a
subsequent diagnostic procedure can be a complementary diagnostic procedure if
the first or preceding procedures, in conjunction with clinical information,
cannot provide an accurate diagnosis. Usually, preference of a procedure over
others depends upon availability, a patient's tolerance, and/or the treating
practitioner's familiarity with the procedure.
4.1 IMAGING STUDIES are generally
accepted, well-established and widely used diagnostic procedures. When
indicated, imaging studies can be utilized for further evaluation of the
Cervical spine, based upon the mechanism of injury, symptoms, and patient
history. Prudent choice of a single diagnostic study, a complementary
combination of studies, or a proper sequential order of complementary studies
will help ensure maximum diagnostic accuracy and minimize adverse effect to the
patient. When the findings of the diagnostic imaging and testing procedures are
not consistent with the clinical examination, the clinical findings should have
preference.
The studies
below are listed in frequency of use, not importance:
4.1.1 Magnetic Resonance Imaging (MRI): is
the imaging study of choice for most abnormalities of the cervical spine. MRI
is useful in suspected nerve root compression, in myelopathy to evaluate the
spinal cord and/or masses, infections such as epidural abscesses or disc space
infection, bone marrow involvement by metastatic disease, and/or suspected disc
herniation or cord contusion following severe neck injury. MRI should be
performed immediately if there is a question of infection or metastatic disease
with cord compression. MRI is contraindicated in patients with certain
implanted devices. In general, the high field, conventional, MRI provides better
resolution. A lower field scan may be indicated when a patient cannot fit into
a high field scanner or is too claustrophobic despite sedation. Inadequate
resolution on the first scan may require a second MRI using a different
technique. All questions in this regard should be discussed with the MRI center
and/or radiologist.
4.1.2 Computed Axial Tomography (CT)
provides excellent visualization of bone and is used to further evaluate bony
masses and suspected fractures and joints not clearly identified on radiographic
evaluation. It may sometimes be done as a complement to MRI scanning to better
delineate bony osteophyte formation in the neural foramen. Instrument-scatter
reduction software provides better resolution when metallic artifact is of
concern.
4.1.3 Myelography is the injection of
radiopaque material into the spinal subarachnoid space, with x-rays then taken
to define anatomy. It may be used as a pre-surgical diagnostic procedure to
obtain accurate information of characteristics, location, and spatial
relationships among soft tissue and bony structures. The use of small needles
and a less toxic, water-soluble, nonionic contrast is recommended.
4.1.4 CT Myelogram provides more detailed
information about relationships between neural elements and surrounding
anatomy.
4.1.5 Bone Scan (Radioisotope Bone Scanning)
is generally accepted, well established, and widely used. Bone scanning is more
sensitive but less specific than MRI. 99mTechnetium diphosphonate uptake
reflects osteoblastic activity and may be useful in diagnosing
metastatic/primary bone tumors, stress fractures, osteomyelitis, and
inflammatory lesions, but cannot distinguish between these entities.
4.1.6 Other Radioisotope Scanning: Indium
and gallium scans are generally accepted, well-established, and widely used
procedures usually to help diagnose lesions seen on other diagnostic imaging
studies. 67Gallium citrate scans are used to localize tumor, infection, and abscesses.
111Indium-labeled leukocyte scanning is utilized for localizing infection or inflammation.
4.1.7 Dynamic [Digital] Fluoroscopy:
Dynamic [Digital] Fluoroscopy of the Cervical spine measures the motion of
intervertebral segments using a videofluoroscopy unit to capture images as the
subject performs Cervical flexion and extension, storing the anatomic motion of
the spine in a computer. Currently it is not recommended for use in the
diagnosis of Cervical instability, since there is limited information on normal
segmental motion for the age groups commonly presenting with Cervical pain, and
diagnostic criteria for specific spinal conditions are not yet defined. No
studies have yet demonstrated predictive value in terms of standard operative
and non-operative therapeutic outcomes.
4.1.8 Diagnostic Spinal Ultrasound is not
recommended in the Cervical, Thoracic and Lumbar Spine
4.2 OTHER TESTS The following
diagnostic procedures in this subsection are listed in alphabetical order, not
by importance:
4.2.1 Electrodiagnostic Testing:
4.2.1.1 Electromyography (EMG), Nerve Conduction
Studies (NCS) are generally accepted, well-established and widely used
diagnostic procedures. EMG and NCS, when performed and interpreted by a trained
physician/electrophysiologist, may be useful for patients with suspected neural
involvement whose symptoms are persistent or unresponsive to initial
conservative treatments. They are used to differentiate peripheral neural
deficits from radicular and spinal cord neural deficits and to rule out
concomitant myopathy. However, F-Wave Latencies are not diagnostic for
radiculopathy. NCS without needle EMG is not diagnostic for radiculopathy and
therefore is not recommended.
In general, EMG
and NCS are complementary to imaging procedures such as CT, MRI, and/or
myelography or diagnostic injection procedures. Electrodiagnostic studies may
provide useful, correlative neuropathophysiological information that would be
otherwise unobtainable from the radiologic studies discussed above.
4.2.1.2 Portable Automated Electrodiagnostic
Device (also known as Surface EMG) is not a substitute for conventional
diagnostic testing in clinical decision-making, and therefore, is not
recommended.
4.2.1.3 Somatosensory Evoked Potential (SSEP) is
not recommended to identify radiculopathy. It may be used to evaluate
myelopathy and other rare neurological disorders such as neurogenic bladder and
sexual dysfunction.
4.2.1.4 Current Perception Threshold (CPT)
Evaluation may be useful as a screening tool, but its diagnostic efficacy in
the evaluation of industrial Cervical pain has not been determined. Therefore,
CPT is not recommended as a diagnostic tool.
4.2.2 Injections - Diagnostic
4.2.2.1 Description - Diagnostic spinal
injections are generally accepted, well-established procedures. These
injections may be useful for localizing the source of pain, and may have added
therapeutic value when combined with injection of therapeutic medication(s).
4.2.2.2 Indications - Since these procedures are
invasive, less invasive or non-invasive procedures should be considered first.
Selection of patients, choice of procedure, and localization of the level for
injection should be determined by clinical information.
4.2.2.3 The interpretation of the test results
are primarily based on functional change, symptom report, and pain response
(via a recognized pain scale), before and at an appropriate time period after
the injection. The diagnostic significance of the test result should be
evaluated in conjunction with clinical information and the results of other
diagnostic procedures. Injections with local anesthetics of differing duration
may be used to support a diagnosis. In some cases, injections at multiple
levels may be required to accurately diagnose neck pain.
Multiple
injections provided at the same session without staging may seriously dilute
the diagnostic value of these procedures. Practitioners must carefully weigh
the diagnostic value of the procedure against the possible therapeutic value.
4.2.2.4 Special Requirements for Diagnostic
Injections Since multi-planar fluoroscopy during procedures is required to
document technique and needle placement, an experienced physician should
perform the procedure. Permanent images are required to verify needle
placement. The subspecialty disciplines of the physicians performing the
injections may be varied, including, but not limited to: anesthesiology,
radiology, surgery, or physiatry. The practitioner should document hands-on
training through workshops of the type offered by organizations such as the
International Spine Intervention Society (ISIS) and/or completed fellowship
training with interventional training. They must also be knowledgeable in
radiation safety.
4.2.2.5 Specific Diagnostic Injections In
general, relief should last for at least the duration of the local anesthetic
used and should significantly relieve pain and result in functional
improvement. Refer to "Injections - Therapeutic" for information on
specific therapeutic injections.
4.2.2.5.1 Medial Branch Blocks are generally accepted
diagnostic injections, used to determine whether a patient is a candidate for
radiofrequency medial branch neurotomy (also known as facet rhizotomy). To be a
positive diagnostic block, the patient should report a reduction of pain of 50%
or greater relief from baseline or the length of time appropriate for the local
anesthetic used. A separate comparative block on a different date may be
performed to confirm the level of involvement. A comparative block uses
anesthetics of varying lengths of activity.
Frequency and
Maximum Duration: May be repeated once for comparative blocks. Limited to 4
levels
4.2.2.5.2 Transforaminal injections are generally
accepted and useful in identifying spinal pathology. When performed for
diagnosis, small amounts of local anesthetic up to a total volume of 1.0 cc
should be used to determine the level of nerve root irritation. A positive
diagnostic block should result in a positive diagnostic functional benefit and
an 50% reduction in nerve-root generated pain appropriate for the anesthetic
used as measured by accepted pain scales (such as a VAS). The use of a
non-particulate steroid is recommended.
Frequency and
Maximum Duration: Once per suspected level. Limited to three levels. May be
repeated once for confirmation.
4.2.2.5.3 Zygapophyseal (Facet) Blocks: Facet blocks
are generally accepted. They may be used diagnostically to direct functional
rehabilitation programs. A positive diagnostic block should result in a
positive diagnostic functional benefit and an 50% reduction in pain appropriate
for the anesthetic used as measured by accepted pain scales (such as a VAS).
They then may be repeated per the therapeutic guidelines.
Frequency and
maximum Duration: Once per suspected level, limited to three levels.
May be repeated for confirmation.
Atlanto-Axial and Atlanto-Occipital injections are generally accepted for
diagnosis and treatment but do not lend themselves to denervation techniques
owing to variable neuroanatomy. Injection of this articulation is complicated
by the proximity of the vertebral artery, which may be tortuous at the level of
the C1 joint. Inadvertent injection of the vertebral artery may cause
respiratory arrest, seizure, stroke, or permanent neurological sequelae. Only
practitioners skilled in these injections should perform them.
Frequency and
Maximum Duration: Once per side
4.2.3 Provocation Discography
4.2.3.1 Description - Discography is an accepted
diagnostic procedure to identify or refute a discogenic source of pain for
patients who are surgical candidates. Discography should only be performed by
physicians who are experienced and have been proctored in the technique. It is
essential that all indications, pre-conditions, special considerations,
procedures, reporting requirements, and results are carefully and specifically
followed. Results should be interpreted judiciously.
4.2.3.2 Indications - Discography may be
indicated when a patient has a history of functionally limiting, unremitting
Cervical pain of greater than four months duration, with or without arm pain,
which has been unresponsive to all conservative interventions. A patient who
would not consider operative therapeutic intervention is not a candidate for an
invasive nontherapeutic intervention, such as provocation discography.
Discography may
prove useful for the evaluation of the pre-surgical spine, such as
pseudarthrosis,
discogenic pain at levels above or below a prior spinal fusion, annular
tear, or internal
disc disruption.
Discography may
show disc degeneration and annular disruption in the absence of low neck pain.
Discography may also elicit concordant pain in patients with mild and
functionally inconsequential neck pain. Because patients with mild neck pain
should not be considered for invasive treatment, discography should not be
performed on these patients. In symptomatic patients with annular tears on
discography, the side of the tear does not necessarily correlate with the side
on which the symptoms occur. The presence of an annular tear does not
necessarily identify the tear as the pain generator.
Discography may
have a limited place in the work-up of pseudarthrosis. Discography may prove
useful in evaluating the number of Cervical spine levels that might require
fusion. CTDiscography provides further detailed information about morphological
abnormalities of the disc and possible lateral disc herniations.
4.2.3.3 Pre-conditions for provocation
discography include all of the following:
4.2.3.3.1 A patient with functionally limiting,
unremitting neck and/or leg pain of greater than four months duration in whom
conservative treatment has been unsuccessful and in whom the specific diagnosis
of the pain generator has not been made apparent on the basis of other
noninvasive imaging studies (e.g., MRI, CT, plain films, etc.). It is
recommended that discography be reserved for use in patients with equivocal MRI
findings, especially at levels adjacent to clearly pathological levels.
Discography may be more sensitive than MRI or CT in detecting radial annular
tears. However, radial tears must always be correlated with clinical
presentation.
4.2.3.3.2 Patients who are considered surgical
candidates (e.g., symptoms are of sufficient magnitude and the patient has been
informed of the possible surgical options that may be available based upon the
results of discography).
4.2.3.3.3 Informed consent regarding the risks and
potential diagnostic benefits of discography has been obtained.
4.2.3.4 Special Considerations:
4.2.3.4.1 Discography should not be performed by the
physician expected to perform the therapeutic procedure. The procedure should
be carried out by an experienced individual who has received specialized
training in the technique of provocation discography.
4.2.3.4.2 Discography should be performed in a
blinded format that avoids leading the patient with anticipated responses. The
procedure should include one or more disc levels thought to be normal or
non-painful in order to serve as an internal control. The patient should not
know what level is being injected in order to avoid spurious results. Abnormal
disc levels may be repeated to confirm concordance.
4.2.3.4.3 Sterile technique must be utilized.
4.2.3.4.4 Judicious use of light sedation during the
procedure is acceptable, represents the most common practice nationally at the
current time, and is recommended by most experts in the field. The patient must
be awake and able to accurately report pain levels during the provocation
portion of the procedure.
4.2.3.4.5 The discography may be performed using a
manometer to record pressure.
4.2.3.4.6 Intradiscal injection of local anesthetic
may be carried out after the provocation portion of the examination and the
patient's response.
4.2.3.4.7 It is recommended that a post-discogram CT
be considered as it frequently provides additional useful information about
disc morphology or other pathology.
4.2.3.5 Reporting of Discography - In addition
to a narrative report, the discography report should contain a standardized
classification of (a) disc morphology (b) the pain response, and (c) the
pressure at which pain is produced. All results should be clearly separated in
the report from the narrative portion. Asymptomatic annular tears are common
and the concordant pain response is an essential finding for a positive
discogram.
4.2.3.5.1 When discography is performed to identify
the source of a patient's neck pain, both a concordant pain response and
morphological abnormalities must be present at the pathological level prior to
initiating any treatment directed at that level. The patient must be awake
during the provocation phase of the procedure; therefore, sedative medication
must be carefully titrated.
4.2.3.5.2 Reporting of pain response should be consistent
with the operational criteria of the International Spine Intervention Society
(ISIS) Guidelines. The report must include the level of concordance for neck
pain using a 10-point VAS, or similar quantitative assessment. It should be
noted that change in the VAS scale before and after provocation is more
important than the number reported.
4.2.4 Thermography
is an accepted and established procedure, but has no use as a diagnostic test
for Cervical pain and is not recommended.
|
|
Patients
undergoing therapeutic procedure(s) are encouraged to return to modified or
restricted duty during their rehabilitation at the earliest appropriate time.
Refer to "Return-to-Work" in this section for detailed information.
Cessation
and/or review of treatment modalities should be undertaken when no further
significant subjective or objective improvement in the patient's condition is
noted. If patients are not responding within the recommended duration periods,
alternative treatment interventions, further diagnostic studies or
consultations should be pursued. Providers should provide and document
education to the patient. No treatment plan is complete without addressing
issues of individual and/or group patient education as a means of facilitating
self-management of symptoms.
Home therapy is
an important component of therapy and may include active and passive
therapeutic procedures, as well as, other modalities to assist in alleviating
pain, swelling, and abnormal muscle tone.
The following
procedures are listed in alphabetical order.
5.1 ACUPUNCTURE
is an accepted and widely used procedure for the relief of pain and inflammation. The exact mode of action is only partially understood. Western medicine studies suggest that acupuncture stimulates the nervous system at the level of the brain, promotes deep relaxation, and affects the release of neurotransmitters. Acupuncture is commonly used as an alternative or in addition to traditional Western pharmaceuticals. While it is commonly used when pain medication is reduced or not tolerated, it may be used as an adjunct to physical rehabilitation and/or surgical intervention to hasten the return of functional activity. Acupuncture should be performed by MD, DO, DC with appropriate training; or a licensed acupuncturist.
5.1.1 Acupuncture: is the insertion and
removal of filiform needles to stimulate acupoints (acupuncture points). Needles may be inserted,
manipulated, and retained for a period of time.
Acupuncture can be used to reduce pain, reduce inflammation, increase blood flow,
increase
range of motion, decrease the side effect of medication-induced nausea, promote
relaxation in an
anxious patient, and reduce muscle spasm.
Indications
include joint pain, joint stiffness, soft tissue pain and inflammation,
paresthesia, postsurgical pain relief, muscle spasm, and scar tissue pain.
5.1.2 Acupuncture with Electrical Stimulation:
is the use of electrical current (micro-amperage or milli-amperage) on the
needles at the acupuncture site. It is used to increase effectiveness of the needles
by continuous stimulation of the acupoint. Physiological effects (depending on
location and settings) can include endorphin release for pain relief, reduction
of inflammation, increased blood circulation, analgesia through interruption of
pain stimulus, and muscle relaxation.
It is indicated
to treat chronic pain conditions, radiating pain along a nerve pathway, muscle
spasm, inflammation, scar tissue pain, and pain located in multiple sites.
5.1.3 Total Time Frames For Acupuncture and
Acupuncture with Electrical Stimulation: Time frames are not meant to be
applied to each of the above sections separately. The time frames are to be
applied to all acupuncture treatments regardless of the type or combination of
therapies being provided.
Time to produce effect: 3 to 6 treatments
Frequency: 1
to 3 times per week Maximum course duration: 14 treatments (one course). Any of
the above acupuncture treatments may extend longer if objective functional
gains can be
documented or when symptomatic benefits facilitate progression in the patient's
treatment program. An additional course of treatment beyond 14 treatments may
be documented with respect to need and ability to facilitate positive
symptomatic or functional gains. Such care should be re-evaluated and documented
with each series of treatments.
5.1.4 Other Acupuncture Modalities:
Acupuncture treatment is based on individual patient needs and therefore
treatment may include a combination of procedures to enhance treatment effect.
Other procedures may include the use of heat, soft tissue manipulation/massage,
and exercise. Refer to Active Therapy (Therapeutic Exercise) and Passive
Therapy sections (Massage and Superficial Heat and Cold Therapy) for a
description of these adjunctive acupuncture modalities and time frames.
5.2 BIOFEEDBACK is a form of
behavioral medicine that helps patients learn self awareness and
self-regulation skills for the purpose of gaining greater control of their
physiology, such as muscle activity, brain waves, and measures of autonomic
nervous system activity. Electronic instrumentation is used to monitor the
targeted physiology and then displayed or fed neck to the patient visually,
auditorially, or tactilely, with coaching by a biofeedback specialist.
Biofeedback is provided by clinicians certified in biofeedback and/or who have
documented specialized education, advanced training, or direct or supervised
experience qualifying them to provide the specialized treatment needed (e.g.,
surface EMG, EEG, or other).
Treatment is
individualized to the patient's work-related diagnosis and needs. Home practice
of skills is required for mastery and may be facilitated by the use of home
training tapes. The ultimate goal of biofeedback treatment is to normalize
physiology to the pre-injury status to the extent possible, and involves
transfer of learned skills to the workplace and daily life. Candidates for
biofeedback therapy or training must be motivated to learn and practice
biofeedback and self-regulation techniques. Indications for biofeedback include
individuals who are suffering from musculoskeletal injury in which muscle
dysfunction or other physiological indicators of excessive or prolonged stress
response affects and/or delays recovery. Other applications include training to
improve self-management of emotional stress/pain responses such as anxiety,
depression, anger, sleep disturbance, and other central and autonomic nervous
system imbalances. Biofeedback is often used in conjunction with other
treatment modalities.
Time to produce effect: 3 to 4 visits
Frequency: 1 to 2 times per week
Maximum duration: 10 to 12 visits. Treatment
beyond 12 visits must be documented with respect to need,
expectation, and ability to facilitate positive functional gains.
5.3 INJECTIONS - THERAPEUTIC
5.3.1 Therapeutic Spinal Injections: Description
- Therapeutic spinal injections may be used after initial conservative
treatments have been undertaken. Therapeutic injections should, with rare
exceptions, be used only after imaging studies and/or diagnostic injections
have established pathology. Special Considerations - For all injections
(excluding trigger point), multi-planar fluoroscopic guidance during procedures
is required to document technique and needle placement, and should be performed
by a physician experienced in the procedure. Permanent images are required to
verify needle replacement. The subspecialty disciplines of the physicians
performing injections may be varied, including, but not limited to:
anesthesiology, radiology, surgery, or physiatry. The practitioner should
document hands-on training through workshops of the type offered by
organizations such as the International Spine Intervention Society (ISIS)
and/or completed fellowship training in pain medicine with interventional
training. They must also be knowledgeable in radiation safety.
5.3.1.1 Epidural Steroid Injection (ESI)
5.3.1.1.1 Description - Epidural steroid injections
are injections of corticosteroid into the epidural space. The purpose of ESI is
to reduce pain and inflammation in the acute or sub-acute phases of injury. ESI
uses two approaches: transforaminal, interlaminar (midline).
5.3.1.1.2 Needle Placement - Multi-planar
fluoroscopic imaging is required for all epidural steroid injections. Contrast
epidurograms allow one to verify the flow of medication into the epidural
space. Permanent images are required to verify needle replacement.
5.3.1.1.3 Indications - There is some evidence that
epidural steroid injections are effective for patients with radicular pain or
radiculopathy (sensory or motor loss in a specific dermatome or myotome). Up to
80% of patients with radicular pain may have initial relief. However, only
25-57% are likely to have excellent long-term relief. Although there is no
evidence regarding the effectiveness of ESI for nonradicular disc herniation,
it is an accepted intervention.
Frequency: One
or more levels can be injected in one session. Whether injections are repeated
depends upon the patient's response to the previous injection. Subsequent
injections may occur. Injections can be repeated if the patient has
demonstrated functional gain and/or pain returns or worsens.
Maximum
duration: Six treatments (a treatment may include injections at one or two
levels) may be done in one year, as per the patient's response to pain and
function. Patients should be reassessed for improvement in pain (as measured by
accepted pain scales) and/or evidence of functional improvement.
5.3.1.2 Zygapophyseal (Facet) Injection
5.3.1.2.1 Description - A generally accepted intra-articular
or pericapsular injection of local anesthetic and corticosteroid.
5.3.1.2.2 Indications- Patients with pain suspected
to be facet mediated in origin. Facet injections may be repeated if they result
in increased documented functional benefit for at least 4 to 6 weeks and/or at
least an 50% initial improvement in pain scales as measured by accepted pain
scales (such as VAS).
Maximum Duration: 4 per level
per year. Maximum three levels
5.3.1.3 Intradiscal Steroid Therapy: Intradiscal
Steroid Therapy consists of injection of a steroid preparation into the
intervertebral disc under fluoroscopic guidance at the time of discography.
There is good evidence that it is not effective in the treatment of suspected
discogenic neck pain and its use is not recommended.
5.3.2 Radio Frequency Medial Branch
Neurotomy/facet rhizotomy:
5.3.2.1 Description -A procedure designed to
denervate the facet joint by ablating the corresponding sensory medial
branches. Continuous percutaneous radiofrequency is the method generally used.
There is good
evidence to support Radio Frequency Medial Branch Neurotomy in the cervical
spine but benefits beyond one year are not yet established. Evidence in the
Cervical spine is conflicting; however, the procedure is generally accepted. In
one study, 60% of patients maintained at least 90% pain relief at 12 months. Radio-frequency
Medial Branch Neurotomy is the procedure of choice over alcohol, phenol, or
cryoablation. Precise positioning of the probe using fluoroscopic guidance is
required. Permanent images should be recorded to verify placement of the
device.
5.3.2.2 Indications -Those patients with
significant, facetogenic pain. Individuals should have met all of the following
indications: Pain of well-documented facet origin, unresponsive to active
and/or passive therapy. It is generally recommended that this procedure not be
performed until three months of conservative therapy have been completed. All
patients should have a successful response to a diagnostic medial nerve branch
block and a separate comparative block. To be a positive diagnostic block the
patient should report a reduction of pain of 50% or greater from baseline for
the length of time appropriate for the local anesthetic used. It is suggested
that this be recorded on a form. A separate comparative block on a different
date may be performed to confirm the level of involvement.
5.3.2.3 Requirements for Repeat Radiofrequency
Medial Branch Neurotomy (or additional-level RF Neurotomy): In some cases pain
may recur. Successful RF Neurotomy usually provides from six to eighteen months
of relief.
Before a repeat
RF Neurotomy is done, a confirmatory medial branch injection should be
performed if the patient's pain pattern presents differently than the initial
evaluation. In occasional patients, additional levels of RF neurotomy may be
necessary. The same indications and limitations apply.
5.3.3 Trigger Point Injections and Dry
Needling Treatment:
5.3.3.1 Description -Trigger point injections
are a generally accepted treatment. Trigger point treatment can consist of dry
needling or injection of local anesthetic, with or without corticosteroid, into
highly localized, extremely sensitive bands of skeletal muscle fibers that
produce local and referred pain when activated. There is no indication for
conscious sedation for patients receiving trigger point injections. The patient
must be alert to help identify the site of the injection.
5.3.3.2 Indications - Trigger point injections
may be used to relieve myofascial pain and facilitate active therapy
and stretching of the affected areas. Trigger point
injections are indicated in those patients where well circumscribed trigger points have
been consistently observed. Generally, these injections are not necessary unless
consistently observed trigger points are not responding to specific,
noninvasive, myofascial
interventions within approximately a 6-week time frame. However, trigger point injections may
be occasionally effective when utilized in the patient with immediate, acute onset of
Cervical pain.
Frequency:
Weekly. Suggest no more than 4 injection sites per session per week to avoid significant post-injection soreness.
Maximum duration: 8 weeks. Occasional patients may require 2 to 4 repetitions
over a 1 to
2 year period.
5.3.4 Prolotherapy: also known as
sclerotherapy consists of a series of injections of hypertonic dextrose, with
or without glycerine and phenol, into the ligamentous structures of the
Cervical Spine. Its proponents claim that the inflammatory response to the
injections will recruit cytokine growth factors involved in the proliferation
of connective tissue, stabilizing the ligaments of the Cervical when these
structures have been damaged by mechanical insults.
There are
conflicting studies concerning the effectiveness of Prolotherapy in the
Cervical. Lasting functional improvement has not been shown. The injections are
invasive, may be painful to the patient, and are not generally accepted or widely
used. Therefore, the use of Prolotherapy for neck pain is not recommended.
5.3.5 Epiduroscopy and Epidural Lysis of
Adhesions: is an investigational treatment of Cervical pain. It involves
the introduction of a fiberoptic endoscope into the epidural space via the
sacral hiatus. With cephalad advancement of the endoscope under direct
visualization, the epidural space is irrigated with saline. Adhesiolysis may be
done mechanically with a fiberoptic endoscope. The saline irrigation is
performed with or without epiduroscopy and is intended to distend the epidural
space in order to obtain an adequate visual field. It is designed to produce
lysis of adhesions, which are conjectured to produce symptoms due to traction
on painful nerve roots. Saline irrigation is associated with risks of elevated
pressures which may impede blood flow and venous return, possibly causing
ischemia of the cauda equina and retinal hemorrhage.
Other
complications associated with instrumented lysis include catheter shearing,
need for catheter surgical removal, infection (including meningitis), hematoma,
and possible severe hemodynamic instability during application. Although
epidural adhesions have been postulated to cause chronic Cervical pain, studies
have failed to find a significant correlation between the level of fibrosis and
pain or difficulty functioning. Studies of epidural lysis demonstrate no
transient pain relief from the procedure. Given the low likelihood of a
positive response, the additional costs and time requirement, and the possible
complications from the procedure, epidural injection, or mechanical lysis, is
not recommended.
Epiduroscopy-directed
steroid injections are also not recommended as there is no evidence to support
an advantage for using an epiduroscope with steroid injections.
5.4 MEDICATIONS use in the treatment
of Cervical injuries is appropriate for controlling acute and chronic pain and
inflammation. Use of medications will vary widely due to the spectrum of
injuries from simple strains to post-surgical healing. All drugs should be used
according to patient needs. A thorough medication history, including use of
alternative and over the counter medications, should be performed at the time
of the initial visit and updated periodically. The patient should be educated
regarding the interaction with prescription and over-the-counter medications as
well as the contents of over-thecounter herbal products.
The use of generic medications is
encouraged. The list below is not all inclusive. It is accepted that
medications not on this list may be appropriate for use in the care of the
injured worker.
The following are listed in alphabetical order:
5.4.1 Acetaminophen: is an effective
analgesic with antipyretic but not anti-inflammatory activity. Acetaminophen is
generally well tolerated, causes little or no gastrointestinal irritation, and
is not associated with ulcer formation. Acetaminophen has been associated with
liver toxicity in overdose situations or in chronic alcohol use. Patients may
not realize that many over-the-counter preparations may contain acetaminophen.
The total daily dose of acetaminophen is recommended not to exceed 4 grams per
24-hour period, from all sources, including narcoticacetaminophen combination
preparations.
5.4.2 Muscle Relaxants: are appropriate for
muscle spasm with pain. There is strong evidence that muscle relaxants are more
effective than placebo for providing short-term pain relief in acute Cervical
pain. When prescribing these agents, physicians must seriously consider side
effects of drowsiness or dizziness and the fact that benzodiazepines may be
habit-forming
5.4.3 Narcotics: should be primarily
reserved for the treatment of severe Cervical pain. In mild to moderate cases
of Cervical pain, narcotic medication should be used cautiously on a
case-bycase basis. Adverse effects include respiratory depression, the
development of physical and impaired alertness.
Narcotic
medications should be prescribed with strict time, quantity, and duration
guidelines, and with definitive cessation parameters. Pain is subjective in
nature and should be evaluated using a scale to rate effectiveness of the
narcotic prescribed.
5.4.4 Nonsteroidal Anti-Inflammatory Drugs
(NSAIDs): are useful for pain and inflammation. In mild cases, they may be
the only drugs required for analgesia. There are several classes of NSAIDs, and
the response of the individual injured worker to a specific medication is
unpredictable. For this reason, a range of NSAIDs may be tried in each case
with the most effective preparation being continued. Patients should be closely
monitored for adverse reactions. Administration of proton pump inhibitors,
Histamine 2 Blockers or prostaglandin analog misoprostol along with these
NSAIDs may reduce the risk of duodenal and gastric ulceration. Intervals for
metabolic screening are dependent upon the patient's age, general health status
and should be within parameters listed for each specific medication.
5.4.4.1 Selective Cyclo-oxygenase-2 (COX-2)
Inhibitors COX-2 inhibitors are more recent NSAIDs and differ in adverse side
effect profiles from the traditional NSAIDs. The major advantages of selective
COX-2 inhibitors over traditional NSAIDs are that they have less
gastrointestinal toxicity and no platelet effects. COX-2 inhibitors should not
be first-line for low risk patients who will be using an NSAID short term but
are indicated in select patients for whom traditional NSAIDs are not tolerated.
Serious upper GI adverse events can occur even in asymptomatic patients. Patients
at high risk for GI bleed include those who use alcohol, smoke, are older than
65, take corticosteroids or anti-coagulants, or have a longer duration of
therapy.
5.4.5 Psychotropic/Anti-anxiety/Hypnotic
Agents: may be useful for treatment of mild and chronic pain, dysesthesias,
sleep disorders, and depression. Antidepressant medications, such as tricyclics
and Selective Serotonin Reuptake Inhibitors (SSRIs), are useful for affective
disorder and chronic pain management. Tricyclic antidepressant agents, in low
dose, are useful for chronic pain. Anti-anxiety medications should generally be
limited to short-term use. Combinations of the above agents may be useful. As a
general rule, physicians should access the patient's prior history of substance
abuse or depression prior to prescribing any of these agents. Due to the
habit-forming potential of the benzodiazepines and other drugs found in this
class, they are not routinely recommended.
5.4.6 Tramadol: is useful in relief of
Cervical pain and has been shown to provide pain relief equivalent to that of
commonly prescribed NSAIDs. Although Tramadol may cause impaired alertness, it
is generally well tolerated, does not cause gastrointestinal ulceration, or
exacerbate hypertension or congestive heart failure.
5.5 OCCUPATIONAL REHABILITATION PROGRAMS
5.5.1 Non-Interdisciplinary: These
generally accepted programs are work-related, outcome focused, individualized
treatment programs. Objectives of the program include, but are not limited to,
improvement of cardiopulmonary and neuromusculoskeletal functions (strength,
endurance, movement, flexibility, stability, and motor control functions), patient
education, and symptom relief. The goal is for patients to gain full or optimal
function and return to work. The service may include the time-limited use of
passive modalities with progression to active treatment and/or simulated/ real
work.
5.5.1.1 Work Conditioning/Simulation:
This program may begin once a patient is out of the acute phase of injury and
will be able to tolerate this program. These programs are usually initiated
after the acute phase has been completed and offered at any time throughout the
recovery phase. Work conditioning should be initiated when imminent return of a
patient to modified or full duty is not an option, but the prognosis for
returning the patient to work at completion of the program is at least fair to
good. The need for work place simulation should be based upon the results of a
Functional Capacity Evaluation and/or Jobsite Analysis.
Length of visit: 1 to 4 hours per day.
Frequency: 2 to 5 visits per week Maximum duration: 8 weeks. Participation in a
program beyond six weeks must be documented with
respect to need and the ability to facilitate positive symptomatic or
functional gains.
5.5.1.2 Work Hardening: Work Hardening is
an interdisciplinary program addressing a patient's employability and return to
work. It includes a progressive increase in the number of hours per day that a
patient completes work simulation tasks until the patient can tolerate a full
workday. This is accomplished by addressing the medical, behavioral, physical,
functional, and vocational components of employability and return-to-work. This
can include a highly structured program involving a team approach or can
involve any of the components thereof. The interdisciplinary team should, at a
minimum, be comprised of a qualified medical director who is board certified
with documented training in occupational rehabilitation; team physicians having
experience in occupational rehabilitation; occupational therapist; physical
therapist; case manager; and psychologist. As appropriate, the team may also include:
Chiropractor, RN, Vocational Specialist or Certified Biofeedback Therapist.
Length of visit: Up to 8 hours/day
Frequency: 2 to 5 visits per week Maximum duration: 8 weeks. Participation in a
program beyond six weeks must be documented with
respect to need and the ability to facilitate positive symptomatic or
functional gains.
5.5.1.3 Spinal Cord Programs: Spinal Cord
Systems of Care provide coordinated, casemanaged, and integrated service for
people with spinal cord dysfunction, whether due to trauma or disease. The
system includes an inpatient component in an organization licensed as a
hospital and an outpatient component. Each component endorses the active
participation and choice of the persons served throughout the entire program.
The Spinal Cord
System of Care also provides or formally links with key components of care that
address the lifelong needs of the persons served. This can include a highly
structured program involving a team approach or can involve any of the
components thereof. The interdisciplinary team should, at a minimum, be
comprised of a qualified medical director who is board certified and trained in
rehabilitation, a case manager, occupational therapy, physical therapy,
psychologist, rehabilitation RN and MD, and therapeutic recreation specialist.
As appropriate, the team may also include: rehabilitation counselor,
respiratory therapist, social worker, or speech-language pathologist.
Time frame
durations for any spinal cord program should be determined based upon the
extent of the patient's injury and at the discretion of the rehabilitation
physician in charge.
5.6 Cervical ORTHOTICS Primary
principles and objectives of the application of cervical orthosis include:
- aid in spinal
stability when soft tissues or osteoligamentous structures cannot sufficiently
perform their role as spinal stabilizers; and
- restrict spinal
segment movement after acute trauma or surgical procedure.
- control of the
position through the use of control forces;
- application of
corrective forces to abnormal curvatures;
In cases of
traumatic cervical injury, the most important objective is the protection of
the spinal cord and nerve root.
5.6.1 Cervical Supports:
5.6.1.1 Soft Collars are well-tolerated
by most patients cervical supports may provide symptomatic relief of pain and
movement reduction in cases of acute cervical conditions. The injured worker
should be advised of the potential harm from using a cervical support for a
period of time greater than that which is prescribed. Harmful effects include
deconditioning of the musculature, skin irritation, and general discomfort.
5.6.1.2 Rigid Collars, such as a
Philadelphia or Miami Orthosis, are useful post-operative or in emergency
situations. These collars restrict flexion and extension motion, and to a
lesser degree, lateral bending and rotation. Duration of wear is dependent upon
the physician and degree of cervical healing but is generally not used beyond 8
weeks.
5.6.1.3 Cervicothoracic Orthosis: such as
Yale and sternal occipital mandibular immobilization (SOMI) type braces,
restrict flexion and extension motion to a fuller degree than the Philadelphia
collar and to a better degree lateral bending and rotation. Not recommended in
sprain or strain type injuries.
5.6.1.4 Halo Devices: are used in the
treatment of cervical fracture, dislocation, and instability at the discretion
of the treating surgeon. Refer to Halo Devices in the Operative Treatment
section.
5.6.1.5 Other Orthosis Devices and Equipment:
Special orthosis or equipment may have a role in the rehabilitation of a
cervical injury such as those injuries to a cervical nerve root resulting in
upper extremity weakness or a spinal cord injury with some degree of
paraparesis or tetraparesis. Use of such devices would be in a structured
rehabilitation setting as part of a comprehensive rehabilitation program.
5.7 PATIENT EDUCATION No treatment
plan is complete without addressing issues of individual and/or group patient
education as a means of prolonging the beneficial effects of treatment, as well
as facilitating self-management of symptoms and injury prevention. The patient
should be encouraged to take an active role in the establishment of functional
outcome goals. They should be educated on their specific injury, assessment
findings, and plan of treatment. Instruction on proper body mechanics and
posture, positions to avoid, self-care for exacerbation of symptoms, and home
exercise should also be addressed.
Time to produce effect: Varies with individual patient
Frequency: Should occur at every visit.
5.8 RESTRICTION OF ACTIVITIES There
is some evidence to support the continuation of normal daily activities as the
recommended treatment for acute and chronic cervical injuries without
neurologic symptoms. Complete work cessation should be avoided, if possible,
since it often further aggravates the pain presentation. Modified
return-to-work is almost always more efficacious and rarely contraindicated in
the vast majority of injured workers with cervical spine injuries.
5.9 RETURN-TO-WORK Early
return-to-work should be a prime goal in treating occupational injuries given
the poor return-to-work prognosis for an injured worker who has been out of
work for more than six months. It is imperative that the patient be educated
regarding the benefits of return-to-work, work restrictions, and follow-up if
problems arise. When attempting to return a patient to work after a specific
injury, clear objective physical capabilities of the injured worker should be outlined
on the appropriate form. An accurate job description with detailed physical
duty requirements is often necessary to assist the physician in making
return-to-work recommendations.
Employers
should be prepared to offer transitional work. This may consist of temporary
work in a less demanding position, return to the regular job with restrictions,
or gradual return to the regular job. Company policies which encourage
return-to-work with positive communication are most likely to have decreased
worker disability. When appropriate a Jobsite Analysis may be necessary.
Return-to-work is defined as any work or duty that the patient is able to
perform safely. It may not be the patient's regular work. Due to the large
spectrum of injuries of varying severity and varying physical demands in the
work place, it is not possible to make specific return-to-work guidelines for
each injury.
5.9.1 Compliance with Activity Restrictions:
In some cases, compliance with restriction of activity levels may require a
complete job site evaluation, a functional capacity evaluation (FCE) or other
special testing.
5.10 THERAPY - PASSIVE Most of the
following passive therapies and modalities are generally accepted methods of
care for a variety of work-related injuries. Passive therapy includes those
treatment modalities that do not require energy expenditure on the part of the
patient. They are principally effective during the early phases of treatment
and are directed at controlling symptoms such as pain, inflammation and swelling
and to improve the rate of healing soft tissue injuries. They should be used
adjunctively with active therapies such as postural stabilization and exercise
programs to help control swelling, pain, and inflammation during the active
rehabilitation process.
Please refer to
General Guideline Principles, Active Interventions. Passive
therapies may be used intermittently as a therapist deems appropriate or
regularly if there are specific goals with objectively measured functional
improvements during treatment.
Generally,
passive interventions are viewed as a means to facilitate progress in an active
rehabilitation program with concomitant attainment of objective functional
gains. All rehabilitation programs must incorporate "Active
Interventions" no later than twelve visits or three weeks after the onset
of treatment. Reimbursement for passive modalities only after the first twelve
visits or three weeks of treatment without clear evidence of Active
Interventions will require supportive documentation.
On occasion,
specific diagnoses and post-surgical conditions may warrant durations of
treatment beyond those listed as "maximum." Factors such as
exacerbation of symptoms, re-injury, interrupted continuity of care, and
co-morbitities may also extend durations of care. Specific goals with
objectively measured functional improvement during treatment must be cited to
justify extended durations of care. It is recommended that, if no functional
gain is observed after the number of treatments under "time to produce
effect" have been completed; alternative treatment interventions, further
diagnostic studies, or further consultations should be pursued.
The following
passive therapies are listed in alphabetical order:
5.10.1 Electrical Stimulation (Unattended and
Attended): is an accepted treatment. Once applied, unattended electrical
stimulation requires minimal on-site supervision by the provider. Indications
include pain, inflammation, muscle spasm, atrophy, decreased circulation, and
the need for osteogenic stimulation. A home unit should be purchased if
treatment is effective and frequent use is recommended.
Time to produce
effect: 2 to 4 treatments
Maximum duration: 24 visits
5.10.2 Iontophoresis: is an accepted
treatment which consists of the transfer of medication, including, but not
limited to, steroidal anti-inflammatories and anesthetics, through the use of
electrical stimulation. Indications include pain (Lidocaine), inflammation
(hydrocortisone, salicylate), edema (mecholyl, hyaluronidase, salicylate),
ischemia (magnesium, mecholyl, iodine), muscle spasm (magnesium, calcium),
calcific deposits (acetate), scars, and keloids (sodium chloride, iodine,
acetate).
Time to produce effect: 1 to 4
treatments Frequency: 3 times per week with at least 48 hours between
treatments Maximum duration: 8 visits per body region
5.10.3 Manipulation: Is generally accepted,
well-established and widely used therapeutic intervention for Cervical pain.
Manipulative Treatment (not therapy) is defined as the therapeutic application
of manually guided forces by an operator to improve physiologic function and/or
support homeostasis that has been altered by the injury or occupational
disease, and has associated clinical significance.
High velocity, low amplitude (HVLA)
technique, chiropractic manipulation, osteopathic
manipulation, muscle energy techniques, counter strain, and non-force
techniques are all types of
manipulative treatment. This may be applied by osteopathic physicians (D.O.),
chiropractors
(D.C.), properly trained physical therapists (P.T.), properly trained
occupational therapist (O.T.), or
properly trained medical physicians.
Under these different types of
manipulation exist many subsets of different techniques that can be
described as a) direct- a forceful engagement of a restrictive/pathologic
barrier, b) indirect- a
gentle/non-forceful disengagement of a restrictive/pathologic barrier, c) the
patient actively assists
in the treatment and d) the patient relaxing, allowing the practitioner to move
the body tissues.
When the proper diagnosis is made and coupled with the appropriate technique,
manipulation has
no contraindications and can be applied to all tissues of the body.
Pre-treatment assessment
should be performed as part of each manipulative treatment visit to ensure that
the correct
diagnosis and correct treatment is employed.
High velocity, low amplitude (HVLA)
manipulation is performed by taking a joint to its end range of
motion and moving the articulation into the zone of accessory joint movement,
well within the limits
of anatomical integrity. Indications for manipulation include joint pain,
decreased joint motion, and
joint adhesions. Contraindications to HVLA manipulation include joint
instability, fractures, severe osteoporosis, infection, metastatic cancer, active inflammatory
arthritides, aortic aneurysm, and
signs of progressive neurologic deficits.
Time to produce effect for all types of manipulative
treatment: 1 to 6 treatments.
Frequency: Up to 3 times per week for the first 4 weeks as indicated by the
severity of involvemen and the desired effect, then up to 2
treatments per week for the next 4 weeks. For further
treatments, twice per week or less to maintain function.
Maximum duration: 30 visits. Extended durations of care beyond what is
considered "maximum" may be necessary in cases of re-injury,
interrupted continuity of care, exacerbation of symptoms,
and in those patients with co-morbidities. Refer to the Chronic Pain Guidelines
for care beyond 6
months.
The combination of 97140 plus either
CMT or OMT code is equal to one visit when performed on the same day. Any
combination of manual therapeutic intervention exceeding 36 visits (not units)
need to go to UR.
5.10.3.1 Mobilization (Joint) / Manipulation:
Mobilization is passive movement involving oscillatory motions to the involved
joints. The passive mobility is performed in a graded manner (I, II, III, IV,
or V), which depicts the speed of the maneuver. It may include skilled manual
joint tissue stretching. Indications include the need to improve joint play,
improve intracapsular arthrokinematics, or reduce pain associated with tissue
impingement.
Time to produce effect: 4 to 6
treatments Frequency: 2 to 3 times per week Maximum duration: 36 visits (CPT
codes 97124 and 97140 cannot exceed 36 visits in combination).
5.10.4 Massage - Manual or Mechanical:
Massage is manipulation of soft tissue with broad ranging relaxation and
circulatory benefits. This may include techniques that include pressing,
lifting, rubbing, pinching of soft tissues by, or with, the practitioner's
hands. Indications include edema (peripheral or hard and non-pliable edema),
muscle spasm, adhesions, the need to improve peripheral circulation and range
of motion, or to increase muscle relaxation and flexibility prior to exercise.
In sub-acute
Cervical pain populations there is good evidence that massage can increase
function when combined with exercise and patient education. Some studies have
demonstrated a decrease in provider visits and pain medication use with
combined therapy. One study indicated improved results with acupressure
massage. It is recommended that all massage be performed by trained,
experienced therapists and be accompanied by an active exercise program and
patient education. In contrast to the sub-acute population, massage is a
generally accepted treatment for the acute Cervical pain population, although
no studies have demonstrated its efficacy for this set of patients.
Time to produce effect: Immediate
Frequency: 1 to 3 times per week Maximum duration: 12 visits (CPT codes 97124
and 97140 cannot exceed 36 visits in combination).
5.10.5 Mobilization (Joint): is a generally
well-accepted treatment. Mobilization is passive movement involving oscillatory
motions to the vertebral segment(s). The passive mobility is performed in a
graded manner (I, II, III, IV, or V), which depicts the speed and depth of
joint motion during the maneuver. For further discussion on Level V joint
mobilization please see section on HVLA manipulation [Refer to section 12. d.].
It may include skilled manual joint tissue stretching.
Indications
include the need to improve joint play, segmental alignment, improve
intracapsular arthrokinematics, or reduce pain associated with tissue
impingement. Mobilization should be accompanied by active therapy. For Level V
mobilization contraindications include joint instability, fractures, severe
osteoporosis, infection, metastatic cancer, active inflammatory arthritides,
aortic aneurysm, and signs of progressive neurologic deficits.
Time to produce effect for all types of manipulative
treatment: 1 to 6 treatments.
Frequency: Up to 3 times per week for the first 4
weeks as indicated by the severity of involvement and the desired effect, then
up to 2 treatments per week for the next 4 weeks. For further treatments, twice
per week or less to maintain function.
Maximum duration: 36 visits. Extended
durations of care beyond what is considered "maximum" may be
necessary in cases of re-injury, interrupted continuity of care, exacerbation
of symptoms, and in those patients with comorbitities. Refer to the Chronic
Pain Guidelines for care beyond 6 months.
RE-EVALUATE TREATMENT EVERY 3 TO 4
WEEKS If a given treatment or modality is not producing positive results
within 3 to 4 weeks, the treatment may be either modified or discontinued. Reconsideration of
diagnosis should also occur in the event of poor response to a seemingly
rational intervention. CPT codes 97124 and 97140 cannot exceed 36 visits in
combination
5.10.6 Mobilization (Soft Tissue): is a generally
well-accepted treatment. Mobilization of soft tissue is the skilled application
of muscle energy, strain/counter strain, myofascial release, manual trigger
point release, and manual therapy techniques designed to improve or normalize
movement patterns through the reduction of soft tissue pain and restrictions.
These can be interactive with the patient participating or can be with the patient
relaxing and letting the practitioner move the body tissues. Indications include muscle spasm around a joint,
trigger points, adhesions, and neural compression. Mobilization should be
accompanied by active therapy. Maximum duration: 36 visits re-evaluate
treatment every 3 to 4 weeks if a given treatment or modality is not producing
positive results within 3 to 4 weeks, the treatment may be either modified or
discontinued. Reconsideration of diagnosis should also occur in the event of
poor response to a seemingly rational intervention. CPT codes 97124
and 97140 cannot exceed 36 visits in combination.
5.10.7 Short-Wave Diathermy: is an accepted
treatment which involves the use of equipment that exposes soft tissue to a
magnetic or electrical field. Indications include enhanced collagen
extensibility before stretching, reduced muscle guarding, reduced inflammatory
response, and enhanced re-absorption of hemorrhage/hematoma or edema. It is an
accepted modality as an adjunct to acupuncture or situation where other forms
of contact superficial heat are contraindicated.
5.10.8 Superficial Heat and Cold Therapy
(excluding Infrared Therapy): is a generally accepted treatment.
Superficial heat and cold are thermal agents applied in various manners that
lower or raise the body tissue temperature for the reduction of pain,
inflammation, and/or effusion resulting from injury or induced by exercise.
Includes application of heat just above the surface of the skin at acupuncture
points. Indications include acute pain, edema and hemorrhage, need to increase
pain threshold, reduce muscle spasm, and promote stretching/flexibility. Cold
and heat packs can be used at home as an extension of therapy in the clinic
setting.
Time to produce effect: Immediate
Frequency: 2 to 5 times per week
Maximum duration: 12 visits with maximum visits 1 per day.
5.10.9 Traction-Manual: is an accepted
treatment and an integral part of manual manipulation or joint mobilization.
Indications include decreased joint space, muscle spasm around joints, and the
need for increased synovial nutrition and response. Manual traction is
contraindicated in patients with tumor, infection, fracture, or fracture
dislocation.
5.10.10 Traction-Mechanical:
Traction modalities are contraindicated in patients with tumor, infections,
fracture, or fracture dislocation. Non-oscillating inversion traction methods
are contraindicated in patients with glaucoma or hypertension. Motorized
traction/decompression devices are included and billed as mechanical traction
(i.e. VAX-D, DRX9000, etc.). A home Cervical traction unit can be purchased if
proves effective and the home unit can provide a similar treatment.
Time to produce effect: 1 to 3 sessions up to 30
minutes. If response is negative after 3 treatments, discontinue this modality.
Frequency: 2 to 3 times per week. A home Cervical traction unit can be
purchased if therapy proves effective.
Maximum duration: 24 visits
5.10.11 Transcutaneous
Electrical Nerve Stimulation (TENS): is a generally accepted treatment.
TENS should include at least one instructional session for proper application
and use. Indications include muscle spasm, atrophy, and decreased circulation
and pain control. Minimal TENS unit parameters should include pulse rate, pulse
width and amplitude modulation. Consistent, measurable functional improvement
should be documented prior to the purchase of a home unit.
Time to produce
effect: Immediate
Frequency: Variable
5.10.12 Ultrasound
(Including Phonophoresis): is an accepted treatment. Ultrasound uses sonic
generators to deliver acoustic energy for therapeutic thermal and/or
non-thermal soft tissue effects. Indications include scar tissue, adhesions,
collagen fiber and muscle spasm, and the need to extend muscle tissue or
accelerate the soft tissue healing. Ultrasound with electrical stimulation is
concurrent delivery of electrical energy that involves dispersive electrode
placement. Indications include muscle spasm, scar tissue, pain modulation, and
muscle facilitation. Phonophoresis is the transfer of medication to the target
tissue to control inflammation and pain through the use of sonic generators.
These topical medications include, but are not limited to, steroidal anti-inflammatory
and anesthetics. Time to produce effect: 6 to 15 treatments
Frequency: 3
times per week
Maximum duration: 24 visits
5.11 THERAPY-ACTIVE The following active
therapies are widely used and accepted methods of care for a variety of
work-related injuries. They are based on the philosophy that therapeutic
exercise and/or activity are beneficial for restoring flexibility, strength,
endurance, function, range of motion, and can alleviate discomfort. Active
therapy requires an internal effort by the individual to complete a specific
exercise or task. This form of therapy requires supervision from a therapist or
medical provider such as verbal, visual, and/or tactile instruction(s). At
times, the provider may help stabilize the patient or guide the movement
pattern but the energy required to complete the task is predominately executed
by the patient.
Patients should be instructed to
continue active therapies at home as an extension of the treatment process in
order to maintain improvement levels. Follow-up visits to reinforce and monitor
progress and proper technique are recommended. Home exercise can include
exercise with or without mechanical assistance or resistance and functional
activities with assistive devices.
The following active therapies are
listed in alphabetical order:
5.11.1 Activities of Daily Living (ADL) are
well-established interventions which involve instruction, active-assisted
training, and/or adaptation of activities or equipment to improve a person's
capacity in normal daily activities such as self-care, work re-integration
training, homemaking, and driving.
Time to produce effect: 4 to 5
treatments
Maximum duration: 10 visits
5.11.2 Aquatic Therapy: is a well-accepted
treatment which consists of the therapeutic use of aquatic immersion for
therapeutic exercise to promote strengthening, core stabilization, endurance,
range of motion, flexibility, body mechanics, and pain management. Aquatic
therapy includes the implementation of active therapeutic procedures in a
swimming or therapeutic pool. The water provides a buoyancy force that lessens
the amount of force gravity applies to the body. The decreased gravity effect
allows the patient to have a mechanical advantage and more likely have a
successful trial of therapeutic exercise. The therapy may be indicated for
individuals who:
- Cannot tolerate
active land-based or full-weight bearing therapeutic procedures;
- Require increased
support in the presence of proprioceptive deficit;
- Are at risk of
compression fracture due to decreased bone density;
- Have symptoms
that are exacerbated in a dry environment;
- Would have a
higher probability of meeting active therapeutic goals than in a land-based
environment. The pool should be large enough to allow full
extremity range of motion and fully erect posture. Aquatic vests, belts and
other devices can be used to provide stability, balance, buoyancy, and
resistance.
Time to produce effect: 4 to 5
treatments
Frequency: 3 to 5 times per week
Maximum duration: 18 visits. A
self-directed program is recommended after the supervised aquatics program has been established,
or, alternatively a transition to a land-based environment exercise program.
5.11.3 Functional Activities: are
well-established interventions which involve the use of therapeutic activity to
enhance mobility, body mechanics, employability, coordination, balance, and
sensory motor integration.
Time to produce effect: 4 to 5
treatments
Frequency: 3 to 5 times per week
Maximum duration: 24 visits Total
number of visit 97110 and 97530 should not exceed 40 visits without preauthorization.
5.11.4 Functional Electrical Stimulation: is
an accepted treatment in which the application of electrical current to elicit
involuntary or assisted contractions of atrophied and/or impaired muscles. It
may be indicated for impaired muscle function due to radiculopathy.
Time to produce effect: 2 to 6
treatments Frequency: 3 times per week
Maximum duration: 24 visits
inclusive of electrical muscle stimulation codes if beneficial provide with
home unit.
5.11.5 Neuromuscular Re-education: is a
generally accepted treatment. It is the skilled application of exercise with
manual, mechanical, or electrical facilitation to enhance strength; movement
patterns; neuromuscular response; proprioception, kinesthetic sense and
coordination; education of movement, balance, and posture. Indications include
the need to promote neuromuscular responses through carefully timed
proprioceptive stimuli, to elicit and improve motor activity in patterns
similar to normal neurologically developed sequences, and improve Neuromotor
response with independent control.
Time to produce effect: 2 to 6
treatments
Frequency: 3-5 times per week
Maximum duration: 36 visits
5.11.6 Therapeutic Exercise: is a generally
well-accepted treatment. Therapeutic exercise, with or without mechanical
assistance or resistance, may include isoinertial, isotonic, isometric and
isokinetic types of exercises. Indications include the need for cardiovascular
fitness, reduced edema, improved muscle strength, improved connective tissue
strength and integrity, increased bone density, promotion of circulation to
enhance soft tissue healing, improvement of muscle recruitment, improved
proprioception, and coordination, and increased range of motion. Therapeutic
exercises are used to promote normal movement patterns, and can also include
complementary/ alternative exercise movement therapy (with oversight of a
physician or appropriate healthcare professional).
5.11.7 Spinal Stabilization: is a generally
well-accepted treatment. The goal of this therapeutic program is to strengthen
the spine in its neural and anatomic position. The stabilization is dynamic
which allows whole body movements while maintaining a stabilized spine. It is
the ability to move and function normally through postures and activities
without creating undue vertebral stress
Time to produce effect: 2 to 6
treatments
Frequency: 3 to 5 times per week
Maximum duration: 36 visits Total
number of visits of 97110 & 97530 may not exceed 40 visits without preauthorization.
5.12 VOCATIONAL
REHABILITATION is a generally accepted intervention. Initiation of
vocational rehabilitation requires adequate evaluation of patients for
quantification of highest functional level, motivation, and achievement of
maximum medical improvement. Vocational rehabilitation may be as simple as
returning to the original job or as complicated as being retrained for a new
occupation. It may also be beneficial for full vocational rehabilitation to be
started if it is evident that the injured worker will be unable to
return to his/her previous occupation. A positive goal and direction may aid
the patient in decreasing stress and depression, and promote optimum
rehabilitation.
|
|
All operative
interventions should be based on a positive correlation with clinical findings,
the natural history of the disease, the clinical course, and diagnostic tests.
A comprehensive assimilation of these factors should have led to a specific
diagnosis with positive identification of the pathologic condition(s). It is
imperative for the clinician to rule out non-physiologic modifiers of pain
presentation, or non-operative conditions mimicking radiculopathy or
instability (peripheral compressive neuropathy, chronic soft tissue injuries,
and psychological conditions), prior to consideration of elective surgical
intervention. Early intervention may be required in acute incapacitating pain
or in the presence of severe or progressive neurological deficits. Patients who
are not candidates for or refuse surgical treatment should be treated with
non-operative therapy as indicated.
Operative
treatment is indicated when the natural history of surgically treated lesions
is better than the natural history for non-operatively treated lesions. All
patients being considered for surgical intervention should first undergo a
comprehensive neuromusculoskeletal examination to identify mechanical pain
generators that may respond to non-surgical techniques, or may be refractory to
surgical intervention. In situations
requiring the possible need for reoperation, and spinal fusions or total disc
replacements over two levels, a second opinion may be necessary.
Interdisciplinary interventions should be strongly considered post-operatively
in patients not making functional progress within expected time
6.1 General Recommendations -If
cervical fusion is being considered, it is recommended that the injured worker
be encouraged to quit or decrease smoking for at least two weeks prior to
surgery and during the time of healing. Because smokers have a higher risk of
non-union and higher post-operative costs, it is recommended that insurers
cover a smoking cessation program peri-operatively.
6.2 General Indications for Surgery -Operative
intervention should be considered and a consultation obtained when improvement
of symptoms has plateaued and the residual symptoms of pain and functional
disability are unacceptable at the end of six weeks of treatment, or at the end
of longer duration of non-operative intervention for debilitated patients with
complex problems. Choice of hardware instrumentation is based on anatomy, the
patient's pathology, and surgeon's experience and preference.
6.3 Specific Indications include:
6.3.1 For Patients with Myelopathy:
immediate surgical evaluation and treatment is indicated.
6.3.2 For Patients with Cervical Radiculopathy:
6.3.2.1 Early intervention may be required for
acute incapacitating pain or in the presence of severe or progressive neurological
deficits.
6.3.2.2 Persistent or recurrent arm pain with
functional limitations, unresponsive to conservative treatment after six weeks;
or
6.3.2.3 Progressive functional neurological
deficit; or
6.3.2.4 Static neurological deficit associated
with significant radicular pain; and
6.3.2.5 Confirmatory imaging studies consistent
with clinical findings.
6.3.3 For Patients with Persistent
Non-radicular Cervical Pain: in the absence of a radiculopathy, it is
recommended that a decisive commitment to surgical or nonsurgical interventions
be made by 4 months following injury. The effectiveness of three-level cervical
fusion for non-radicular pain has not been established. In patients with
non-radicular cervical pain for whom fusion is being considered, required
pre-operative indications include all of the following:
6.3.3.1 In general, if the program of
non-operative treatment fails, operative treatment is indicated when:
6.3.3.1.1 Improvement of the symptoms has plateaued,
and the residual symptoms of pain and functional disability are unacceptable at
the end of 6 to 12 weeks of active treatment, or at the end of longer duration
of non-operative programs for debilitated patients with complex problems;
and/or
6.3.3.1.2 Frequent recurrences of symptoms cause
functional limitations even if a non-operative active treatment program
provides satisfactory relief of symptoms, and restoration of function on each
recurrence.
6.3.3.2 Pain generators are adequately defined
and treated; and
6.3.3.3 Physical medicine and manual therapy
interventions are completed
6.3.3.4 X-ray, MRI, or CT/discography
demonstrating disc pathology or spinal instability.
6.3.3.5 Spine pathology is limited to two levels.
6.4 Surgical Procedures include:
6.4.1 Cervical Discectomy with or without
Fusion:
6.4.1.1 Description - Procedure to
relieve pressure on one or more nerve roots or spinal cord. It may be performed
with or without the use of a microscope.
6.4.1.2 Surgical
Indications - Radiculopathy from ruptured disc or spondylosis, spinal
instability, or patients with non-radicular neck pain meeting fusion criteria.
Discectomy alone from a posterior approach may be considered in patients with
pure radicular symptoms from their herniated disc and who have sufficiently
large foramen that disc space collapse is unlikely to further compromise the
nerve root. Failure rates increase with disease at more than two levels.
6.4.1.3 Operative Treatment - Cervical
plating may be used to prevent graft dislodgment and facilitate fusion. It has
the added advantage of eliminating the need for postoperative bracing and
allowing faster functional recovery. Recombinant Human Bone Morphogenetic
Protein (rhBMP-2) is a member of a family of cytokines capable of inducing bone
formation. It is produced from genetically modified cell lines using molecular
cloning techniques. Use of rhBMP-2 in the cervical spine may carry a risk of
swelling and ectopic bone formation which can encroach on neurovascular
structures and on the esophagus. As of the date of adoption the FDA has not
approved its use in the cervical spine. At the time of this guideline, cervical
application of rhBMP-2 is investigational and remains outside the purview of
the guidelines. Prior authorization is required.
6.4.1.4 Post-Operative Therapy - Cervical
bracing may be used. Home programs with instruction in ADLs, sitting, posture,
and a daily walking program should be an early part of the rehabilitation
process. Referral to a formal rehabilitation program, with emphasis on
cervical, scapular, and thoracic strengthening and restoration of ROM is
appropriate and encouraged to expedite a return to higher function. Active
treatment, which patients should have had prior to surgery, will frequently
require a repeat of the sessions previously ordered. The goals of the therapy
program should include instruction in a long-term home-based exercise program.
6.4.1.5 Intervertebral Biomechanical
Device(s) and Use of Code 22851 Code 22851 describes the application of an
intervertebral biomechanical device to a vertebral defect or interspace. Code
22851 should be listed in conjunction with a primary procedure without the use
of modifier 51. The use of 22851 is limited to one instance per single
interspace or single vertebral defect regardless of the number of devices
applied and infers additional qualifying training, experience, sizing, and/or
use of special surgical appliances to insert the biomechanical device.
Qualifying devices include manufactured synthetic or allograft biomechanical
devices, or methyl methacrylate constructs, and are not dependant on a specific
manufacturer, shape, or material of which it is constructed. Qualifying devices
are machine cut to specific dimensions for precise application to an
intervertebral defect. (For example, the use of code 22851 would be appropriate
during a cervical arthrodesis (22554) when applying a synthetic alloy cage, a threaded
bone dowel, or a machine cut hexahedron cortical, cancellous, or cortico
cancellous allograft biomechanical device. Surgeons
utilizing generic non-machined bony allografts or autografts are referred to
code sets 20930-20931, 20936-20938respectively.)
6.4.2 Cervical Corpectomy
6.4.2.1 Description -Removal of a portion
or the entire vertebral body from the front of the spine. May also include
removal of the adjacent discs. Usually involves fusion.
6.4.2.2 Surgical Indications - Single or
two-level spinal stenosis, spondylolisthesis, or severe kyphosis, with cord
compression.
6.4.2.3 Operative Treatment - Neural
decompression, fusion with instrumentation, or halo vest placement to maintain
cervical position. Hemicorpectomy may be done when only a portion of the
vertebral body needs to be resected. Allografts may be used for single bone
graft fusion; however, autografts are generally preferable for multi-level
fusions unless a large strut graft is required.
6.4.2.4 Post-Operative Therapy -
Dependent upon number of vertebral bodies involved, healing time may be longer
than discectomy. Halo vest care is required. Home programs with instruction in
ADLs, sitting, posture, and a daily walking program should be an early part of
the rehabilitation process. Referral to a formal rehabilitation program with
emphasis on cervical, scapular, and thoracic strengthening is appropriate for
most patients once the cervical spine is deemed stable and without
complication. The goals of the therapy program should include instruction in a
long-term home-based exercise program.
6.4.3 Cervical
Laminectomy with or without Foraminotomy or Fusion
6.4.3.1 Description -Surgical removal of
the posterior portion of a vertebrae in order to gain access to the spinal cord
or nerve roots.
6.4.3.2 Surgical Indications - Neural
compression.
6.4.3.3 Operative Treatment - Laminotomy,
partial discectomy, and nerve root decompression.
6.4.3.4 Post-Operative Therapy -Cervical
bracing may be appropriate (usually 6 to 12 weeks with fusion). Home programs
with instruction in ADLs, sitting, posture, and a daily walking program should
be an early part of the rehabilitation process. Referral to a formal
rehabilitation program with emphasis on cervical, scapular, and thoracic
strengthening and restoration of ROM is appropriate for most patients once the
cervical spine is deemed stable and without complication. The goals of the
therapy program should include instruction in a long-term home-based exercise
program
6.4.4 Cervical
Laminoplasty
6.4.4.1 Description -Technique that
increases anterior or posterior dimensions of the spinal canal while leaving
posterior elements partially intact. It may be performed with or without the
use of a microscope.
6.4.4.2 Surgical Indications - Multi-level
disease: cervical spinal stenosis or spondylitic myelopathy. Not indicated in
cervical kyphosis.
6.4.4.3 Operative Treatment - Posterior
approach, with or without instrumentation.
6.4.4.4 Post-Operative Therapy -May
include 4 to 12 weeks of cervical bracing. Home programs with instruction in
ADLs, sitting, posture, and daily walking program should be an early part of
the rehabilitation process. Referral to a formal rehabilitation program with
emphasis on cervical, scapular, and thoracic strengthening and restoration of
ROM is appropriate once the cervical spine is stable and without complication.
Active treatment which patients should have had prior to surgery will
frequently require a repeat of the sessions previously ordered. The goals of
the therapy program should include instruction in a long-term, home-based
exercise program.
6.4.5 Artificial
Cervical Disc Replacement
6.4.5.1 Description - involves the
insertion of a prosthetic device into an intervertebral space from which a
degenerated disc has been removed, sparing only the peripheral annulus. The
endplates are positioned under intraoperative fluoroscopic guidance for optimal
placement in the sagittal and frontal planes. The prosthetic device is designed
to distribute the mechanical load of the vertebrae in a physiologic manner and
maintain range of motion.
6.4.5.2 General Selection Criteria - for
cervical disc replacement includes symptomatic degenerative disc disease. The
patient must also meet fusion surgery criteria, and if the patient is not a
candidate for fusion, a disc replacement procedure should not be considered.
Additionally, the patient should be able to comply with pre-and post-surgery
protocol.
6.4.5.3 The Theoretical Advantage - of
total disc arthroplasty is that it preserves range of motion and physiologic
loading of the disc. This could be an advantage for adults who are physically
active. Studies do not demonstrate a long-term advantage of measured function
or pain over comparison groups undergoing fusion. The longevity of this
prosthetic device has not yet been determined. Significant technical training
and experience is required to perform this procedure successfully. Surgeons
must be well-versed in anterior spinal techniques and should have attended
appropriate training courses, or have undergone training during a fellowship.
Mentoring and proctoring of procedures is highly recommended.
6.5 External Spinal Stimulators Post
Fusion
6.5.1 The following criteria are established for
the medically accepted standard of care when determining applicability for the
use of an external spinal stimulator:
6.5.1.1 Patient has had a previously failed
spinal fusion, and/or
6.5.1.2 Patient is scheduled for revision or
repair of pseudoarthrosis, and/or
6.5.1.3 The patient smokes greater than a pack
of cigarettes per day and is scheduled for spinal fusion
6.5.1.4 The external spinal stimulator is
approved for use in primary spinal fusions, if medical co morbidities increase
the likelihood of non-union
6.5.1.5 The patient is metabolically in poor
health, with other medical co morbidities such as diabetes, Rheumatoid
arthritis, lupus or other illnesses requiring oral steroids or cytotoxic
medications.
6.5.2 Precertification is
required for use of the external spinal stimulator if the planned use falls
outside the above indications.
|
PART G LOWER EXTREMITY TREATMENT GUIDELINES
|
Pursuant to 19 Del.C. §2322C, health care practice guidelines have been adopted and recommended by the Health Care Advisory Panel to guide utilization of health care treatments in workers' compensation including,
but not limited to, care provided
for the treatment of employees by or under the supervision of a licensed health
care provider, prescription drug
utilization, inpatient hospitalization and
length of stay, diagnostic testing, physical therapy, chiropractic care and
palliative care. The health care
practice guidelines apply to all
treatments provided after the effective date of the regulation adopted by the
Department of Labor, May 23, 2008,
and regardless of the date of
injury. Lower Extremity was added to the list of treatment guidelines, effective 6/13/2011. The guidelines are, to the
extent permitted by the most current medical science or applicable science,
based on well-documented
scientific research concerning efficacious treatment for injuries and occupational disease. To the extent that well-documented
scientific research regarding the above is not available at the time of
adoption of the guidelines, or is not available at the time of any revision to
the guidelines, the guidelines have been and
will be based upon the best available information concerning national consensus
regarding best health care
practices in the relevant health care
community. The guidelines, to
the extent practical and consistent with the Act, address treatment of those
physical conditions which occur with the greatest frequency, or which require
the most expensive treatments, for work-related injuries based upon currently
available Delaware
data.
Services
rendered by any health care provider certified pursuant to 19 Del.C. §2322D(a)
to provide treatment or services for injured employees shall be presumed, in
the absence of contrary evidence, to be reasonable and necessary if such
treatment or service conforms to the most current version of the Delaware
health care practice guidelines.
Services
rendered outside the Guidelines or variation in treatment recommendations from
the Guidelines may represent acceptable medical care, be considered reasonable
and necessary treatment and, therefore, determined to be compensable, absent
evidence to the contrary, and may be payable in accordance with the Fee Schedule
and Statute, accordingly.
Services
provided by any health care provider that is not certified pursuant to 19
Del.C. §2322D(a) shall not be presumed reasonable and necessary unless such
services are pre-authorized by the employer or insurance carrier, subject to
the exception set forth in 19 Del.C. §2322D(b).
Treatment
of conditions unrelated to the injuries sustained in an industrial accident may
be denied as unauthorized if the treatment is directed toward the
non-industrial condition, unless the treatment of the unrelated injury is
rendered necessary as a result of the industrial accident.
The
Health Care Advisory Panel and Department of Labor recognizes that acceptable
medical practice may include deviations from these Guidelines, as individual cases
dictate. Therefore, these Guidelines are not relevant as evidence of a
provider's legal standard of professional care.
In
accordance with the requirements of the Act, the development of the health care
guidelines has been directed by a predominantly medical or other health
professional panel, with recommendations then made to the Health Care Advisory
Panel.
|
|
The principles
summarized in this section are key to the intended implementation of all Delaware
Workers’ Compensation practice guidelines and critical to the reader’s
application of the guidelines in this document.
2.1 EDUCATION of the patient and family, as well as
the employer, insurer, policy makers and the community should be the primary
emphasis in the treatment of lower extremity pain and disability. Currently,
practitioners often think of education last, after medications, manual therapy
and surgery. Practitioners must develop
and implement an effective strategy and skills to educate patients, employers,
insurance systems, policy makers and the community as a whole. An
education-based paradigm should always start with inexpensive communication
providing reassuring information to the patient. More in-depth education currently exists
within a treatment regime employing functional restorative and innovative
programs of prevention and rehabilitation.
No treatment plan is complete without addressing issues of individual
and/or group patient education as a means of facilitating self-management of
symptoms and prevention.
2.2 TREATMENT
PARAMETER DURATION: Time
frames for specific interventions commence once treatments have been initiated,
not on the date of injury. Obviously,
duration will be impacted by patient compliance, as well as co-morbidities and availability
of services. Clinical judgment may
substantiate the need to accelerate or decelerate the time frames discussed in
this document. Causality of symptoms and
dysfunction may occur in a related but different area as a result of the body
compensating for the original injury. This
scenario is particularly true in the case of lower extremity conditions.
2.2.1 Lower extremity injuries are often
not isolated and frequently compensation for injury in one area may result in
symptoms or dysfunction in another area.
Additionally, weakness or dysfunction in an adjacent or otherwise remote
body region may be a predisposing or perpetuating factor for the injured
area. Therefore, treatment applied to
adjacent body regions is often required for the recovery of lower extremity
injuries. The provider’s documentation
should clearly include the rationale for treating adjacent or remote body
regions, as well as include the specific interventions provided.
2.3 ACTIVE INTERVENTIONS emphasizing patient responsibility, such as
therapeutic exercise and/or functional treatment, are generally emphasized over
passive modalities, especially as treatment progresses. Generally, passive interventions are viewed
as a means to facilitate progress in an active rehabilitation program with
concomitant attainment of objective functional gains. All rehabilitation programs must incorporate
“Active Interventions” no later than three weeks after the onset of treatment. Reimbursement for passive modalities only
after the first three weeks of treatment without clear evidence of Active
Interventions will require supportive documentation.
2.4 ACTIVE
THERAPEUTIC EXERCISE PROGRAM
goals should incorporate patient strength, endurance, flexibility,
coordination, and education. This includes
functional application in vocational or community settings.
2.5 POSITIVE PATIENT RESPONSE results are defined
primarily as functional gains that can be objectively measured. Objective functional gains include, but are
not limited to, positional tolerances, range-of-motion (ROM), strength, and endurance, activities
of daily living (ADL), cognition,
psychological behavior, and efficiency/velocity measures that can be
quantified. Subjective reports of pain
and function should be considered and given relative weight when the pain has
anatomic and physiologic correlation.
Anatomic correlation must be based on objective findings.
2.6 RE-EVALUATE TREATMENT EVERY 3 TO 4 WEEKS: If a
given treatment or modality is not producing positive results within 3 to 4
weeks, the treatment should be either modified or discontinued. Reconsideration of diagnosis should also
occur in the event of poor response to a seemingly rational intervention.
2.7 SURGICAL INTERVENTIONS: Surgery
should be contemplated within the context of expected functional outcome and
not purely for the purpose of pain relief.
The concept of “cure” with respect to surgical treatment by itself is
generally a misnomer. All operative
interventions must be based upon positive correlation of clinical findings,
clinical course and diagnostic tests. A
comprehensive assimilation of these factors must lead to a specific diagnosis
with positive identification of pathologic conditions.
2.8 SIX-MONTH
TIME FRAME: The
prognosis drops precipitously for returning an injured worker to work once
he/she has been temporarily totally disabled for more than six months. The emphasis within these guidelines is to
move patients along a continuum of care and return to work within a six-month
time frame, whenever possible. It is
important to note that time frames may not be pertinent to injuries that do not
involve work-time loss or are not occupationally related.
2.9 RETURN-TO-WORK
is therapeutic, assuming the work is not likely to aggravate the basic problem
or increase long-term pain. The
practitioner must provide specific physical limitations per the Physician’s
Form. The following physical limitations should be considered and modified as
recommended: lifting, pushing, pulling,
crouching, walking, using stairs, bending at the waist, awkward and/or
sustained postures, tolerance for sitting or standing, hot and cold
environments, data entry and other repetitive motion tasks, sustained grip,
tool usage and vibration factors. Even
if there is residual chronic pain, return-to-work is not necessarily
contraindicated. The practitioner should
understand all of the physical demands of the patient’s job position before
returning the patient to full duty and should receive clarification of the
patient’s job duties.
2.10 DELAYED
RECOVERY: Strongly
consider a psychological evaluation, if not previously provided, as well as
initiating interdisciplinary rehabilitation treatment and vocational goal
setting, for those patients who are failing to make expected progress 6 to 12
weeks after an injury. A small
percentage of industrially injured patients will not recover within the
timelines outlined in this document despite optimal care. Such individuals may require treatments
beyond the limits discussed within this document, but such treatment will
require clear documentation by the authorized treating practitioner focusing on
functional gains afforded by further treatment and impact upon prognosis.
2.11 GUIDELINES
RECOMMENDATIONS AND INCLUSION OF MEDICAL
EVIDENCE: Guidelines are recommendations based on available evidence
and/or consensus recommendations. Those procedures considered inappropriate,
unreasonable, or unnecessary are designated in the guideline as being “not
recommended.”
|
|
The guidelines recommend the following diagnostic procedures be
considered, at least initially. It is the responsibility of the workers’
compensation carrier to ensure that an accurate diagnosis and treatment plan
can be established. Standard procedures
that should be utilized when initially diagnosing a work-related lower
extremity complaint are listed below.
3.1 HISTORY-TAKING AND PHYSICAL EXAMINATION (Hx & PE)
are generally accepted,
well-established and widely used procedures that establish the foundation/basis
for and dictates subsequent stages of diagnostic and therapeutic
procedures. When findings of clinical
evaluations and those of other diagnostic procedures are not complementing each
other, the objective clinical findings should have preference. The medical records can reasonably document
the following:
3.1.1 History of Present Injury:
3.1.1.1 Mechanism
of injury. This includes details of
symptom onset and progression. It should
include such details as: the activity at
the time of the injury, patient description of the incident, and immediate and
delayed symptoms. The history should elicit as much detail about these
mechanisms as possible.
3.1.1.2 History
of locking, clicking, popping, giving way, acute or chronic swelling,
crepitation, pain while ascending or descending stairs (e.g. handrail used,
‘foot by foot’ instead of ‘foot over foot’) inability to weight bear due to
pain, intolerance for standing or difficulty walking
distances on varied surfaces, difficulty crouching or stooping, and wear
patterns on footwear. Patients may also
report instability or mechanical symptoms.
3.1.1.3 Any history of pain in back as well as joints distal and
proximal to the site of injury. The use of a patient completed pain drawing,
Visual Analog Scale (VAS), is highly recommended, especially during the first
two weeks following injury to assure that all work related symptoms are
addressed.
3.1.1.4 Ability
to perform job duties and activities of daily living.
3.1.1.5 Exacerbating
and alleviating factors of the reported
symptoms. The physician should explore
and report on non-work related as well as, work related activities.
3.1.1.6 Prior
occupational and non-occupational injuries to the same area including specific
prior treatment and any prior bracing devices.
3.1.1.7 Discussion
of any symptoms present in the uninjured extremity.
3.1.2 Past
History:
3.1.2.1 Past
medical history includes neoplasm, gout, arthritis, previous musculoskeletal
injuries, and diabetes;
3.1.2.2 Review
of systems includes symptoms of rheumatologic, neurological,
endocrine, neoplastic, and other systemic diseases;
3.1.2.3 History
of smoking, alcohol use, and substance abuse;
3.1.2.4 History
of corticosteroid use; and
3.1.2.5 Vocational
and recreational pursuits.
3.1.3 Physical
Examination: Examination of a joint should begin with examination of the
uninjured limb and include assessment of the joint above and below the affected
area of the injured limb. Physical
examinations should include accepted tests as described in textbooks or other
references and exam techniques applicable to the joint or region of the body
being examined, including:
3.1.3.1 Visual
inspection;
3.1.3.1.1 Swelling
may indicate joint effusion from trauma, infection or arthritis. Swelling or bruising over ligaments or bones
can indicate possible fractures or ligament damage;
3.1.3.2 Palpation
for joint line tenderness, effusion, and bone or ligament pain;
3.1.3.2.1 Palpation
may be used to assess tissue tone and contour; myofascial trigger points; and
may be graded for intensity of pain. Palpation may be further divided into
static and motion palpation. Static
palpation consists of feeling bony landmarks and soft tissue structures and
consistency. Motion palpation is
commonly used to assess joint movement patterns and identify joint dysfunction;
3.1.3.3 Assessment
of activities of daily living including gait abnormalities, especially after
ambulating a distance and difficulties ascending/descending stairs;
3.1.3.3.4 Assessment
of activities such as the inability to crouch or stoop, may give important
indications of the patient’s pathology and restrictions.
3.1.3.4 Range-of-motion
and quality-of-motion should be assessed actively and passively;
3.1.3.5 Strength;
3.1.3.6 Joint
stability;
3.1.3.7 Hip
exam;
3.1.3.7.1 In
general, multiple tests are needed to reliably establish a clinical diagnosis.
Spinal pathology and groin problems should always be considered and ruled out
as a cause of pain for patients with hip symptomatology. The following lists commonly performed tests:
3.1.3.7.1.1 Flexion-Abduction-External
Rotation (FABER-aka Patrick’s) test is frequently used as a test for sacral
pathology;
3.1.3.7.1.2 Log
roll test may be used to assess iliofemoral joint laxity;
3.1.3.7.1.3 Ober’s is used to test the
iliotibial band;
3.1.3.7.1.4 Greater
trochanter bursitis is aggravated by external rotation and adduction and resisted
hip abduction or external rotation;
3.1.3.7.1.5 Iliopectineal
bursitis may be aggravated by stretching the tendon in hip extension;
3.1.3.7.1.6 Internal
and external rotation is usually painful in osteoarthritis; and
3.1.3.7.1.7 The
maneuvers of flexion, adduction and internal rotation (FADIR) will generally
reproduce pain in cases of labral tears and with piriformis strain/irritation.
3.1.3.8 Knee exam;
3.1.3.8.1 In
general, multiple tests are needed to reliably establish a clinical
diagnosis. The expertise of the
physician performing the exam influences the predictability of the exam
findings. Providers should be aware that
patients with osteoarthritis may have positive pain complaints with various
maneuvers based on their osteoarthritis rather than ligamentous or meniscal
damage. The following partial list contains commonly performed tests:
3.1.3.8.1.1 Bilateral
thigh circumference measurement assesses for quadriceps wasting which may occur
soon after a knee injury. The circumferences of both thighs should be
documented approximately 15 cm above a reference point, either the joint line
or patella.
3.1.3.8.1.2 Anterior
Cruciate Ligament tests:
3.1.3.8.1.2.1 Lachman’s
test;
3.1.3.8.1.2.2 Anterior drawer test;
3.1.3.8.1.2.3 Lateral pivot shift test.
3.1.3.8.1.3 Meniscus tests. Joint line tenderness and effusions are
common with acute meniscal tears.
Degenerative meniscal tears are fairly common in older patients with
degenerative changes and may be asymptomatic.
3.1.3.8.1.3.1 McMurray test;
3.1.3.8.1.3.2 Apley compression test;
3.1.3.8.1.3.3 Medial lateral grind test;
3.1.3.8.1.3.4 Weight-bearing
tests - include Thessaly and Ege’s test.
3.1.3.8.1.4 Posterior
Cruciate Ligament tests:
3.1.3.8.1.4.1 Posterior
drawer test;
3.1.3.8.1.4.2 Extension lag may also be measured
passively by documenting the heel height difference with the patient
prone.
3.1.3.8.1.5 Collateral
Ligaments tests:
3.1.3.8.1.5.1 Medial stress test – A positive test
in full extension may include both medial collateral ligament and cruciate
ligament pathology;
3.1.3.8.1.5.2 Lateral stress test.
3.1.3.8.1.6 Patellar
Instability tests:
3.1.3.8.1.6.1 Apprehension test;
3.1.3.8.1.6.2 J sign;
3.1.3.8.1.6.3 Q angle.
3.1.3.9 Foot
and ankle exam:
3.1.3.9.1 In
general, multiple tests are needed to reliably establish a clinical
diagnosis. The expertise of the
physician performing the exam influences the predictability of the exam
findings. Ankle assessments may include
anterior drawer exam, talar tilt test, external rotation stress test, ankle
ligament stress test and the tibia-fibula squeeze test. Achilles tendon may be assessed with the
Thompson's test. Foot examinations may include, assessment of or for: subtalar,
midtarsal, and metatarsal-phalangeal joints; tarsal tunnel; and posterior
tibial tendon; Morton's neuroma; the piano key test and Lisfranc injury.
3.1.3.10 If
applicable, full neurological exam including muscle atrophy and gait
abnormality.
3.1.3.11 If
applicable to injury, integrity of distal circulation, sensory, and motor
function.
3.2 RADIOGRAPHIC
IMAGING of the lower extremities is a generally
accepted, well-established and widely used diagnostic procedure when specific
indications based on history and/or physical examination are present. It should not be routinely performed. The mechanism of injury and specific
indications for the radiograph should be listed on the request form to aid the
radiologist and x-ray technician. For
additional specific clinical indications, see Section 5.0, Specific Lower
Extremity Injury Diagnosis, Testing and Treatment. Indications for initial imaging include any of
the following:
3.2.1 The
inability to flex knee to 90 degrees or to transfer weight for four steps at
the time of the immediate injury and at the initial visit, regardless of
limping;
3.2.2 Bony
tenderness on any of the following areas: over the head of the fibula; isolated
to the patella; of the lateral or medial malleolus from the tip to the distal 6
cm; at the base of the 5th metatarsal; or at the navicular;
3.2.3 History
of significant trauma, especially blunt trauma or fall from a height;
3.2.4 Age
over 55 years;
3.2.5 History
or exam suggestive of intravenous drug abuse or osteomyelitis;
3.2.6 Pain
with swelling and/or range of motion (ROM) limitation localizing to an area of
prior fracture, internal fixation, or joint prosthesis; or
3.2.7 Unexplained
or persistent lower extremity pain over two weeks.
3.2.7.1 Occult
fractures, especially stress fractures, may not be visible on initial
x-ray. A follow-up radiograph, MRI
and/or bone scan may be required to make the diagnosis.
3.2.7.2 Weight-bearing
radiographs are used to assess osteoarthritis and alignment prior to some
surgical procedures.
3.3 LABORATORY TESTING Laboratory tests are generally accepted, well-established and
widely used procedures. They are,
however, rarely indicated at the time of initial evaluation, unless there is
suspicion of systemic illness, infection, neoplasia, connective tissue
disorder, or underlying arthritis or rheumatologic disorder based on history
and/or physical examination. Laboratory
tests can provide useful diagnostic information. It is recommended that lab diagnostic
procedures be initially considered the responsibility of the workers'
compensation carrier to ensure that an accurate diagnosis and treatment plan
can be established. Tests
include, but are not limited to the following:
3.3.1 Complete
blood count (CBC) with differential can detect infection, blood dyscrasias, and
medication side effects;
3.3.2 Erythrocyte
sedimentation rate, rheumatoid factor, antinuclear antigen (ANA), human
leukocyte antigen (HLA), and
C-reactive protein (CRP) can be used to detect evidence of a rheumatologic,
infection, or connective tissue disorder;
3.3.3 Serum
calcium, phosphorous, uric acid, alkaline phosphatase, and acid phosphatase can
detect metabolic bone disease;
3.3.4 Liver
and kidney function may be performed for prolonged anti-inflammatory use or
other medications requiring monitoring; and
3.3.5 Analysis
of joint aspiration for bacteria, white cell count, red cell count, fat
globules, crystalline birefringence and chemistry to evaluate joint effusion.
3.4. OTHER PROCEDURES
3.4.1 Joint Aspiration is a generally accepted, well-established and
widely used procedure when specifically indicated and performed by individuals
properly trained in these techniques.
This is true at the initial evaluation when history and/or physical
examination are of concern for a septic joint or bursitis and for some acute
injuries. Particularly at the knee,
aspiration of a large effusion can help to decrease pain and speed functional
recovery. Persistent or unexplained
effusions may be examined for evidence of infection, rheumatologic, or
inflammatory processes. The presence of
fat globules in the effusion strongly suggests occult fracture.
3.4.1.1 Risk
factors for septic arthritis include joint surgery, knee arthritis, joint
replacement, skin infection, diabetes, age greater than 80, immunocompromised
states, and rheumatoid arthritis. More
than 50% of patients with septic joints have a fever greater than 37.5 degrees
centigrade and joint swelling. Synovial white counts of greater than 25,000 and
polymorphonuclear cells of at least 90% increase the likelihood of a septic
joint.
3.4.2 Musculoskeletal Ultrasound. The use of diagnostic ultrasound may be
beneficial for guiding injections into the pathologic areas. Ultrasound guided interventional procedure
provides the ability to image soft tissues in real time and can improve safety
and accuracy of needle placement. The
use of ultrasound guided procedures will be at the discretion of the health
care provider.
|
|
One
diagnostic imaging procedure may provide the same or distinctive information as
obtained by other procedures. Therefore,
prudent choice of procedure(s) for a single diagnostic procedure, a complementary
procedure in combination with other procedures(s), or a proper sequential order
in multiple procedures will ensure maximum diagnostic accuracy; minimize
adverse effect to patients and cost effectiveness by avoiding duplication or
redundancy.
All diagnostic imaging procedures
have a significant percentage of specificity and sensitivity for various
diagnoses. None is specifically
characteristic of a certain diagnosis.
Clinical information obtained by history taking and physical examination
should be the basis for selection and interpretation of imaging procedure
results.
When
a diagnostic procedure, in conjunction with clinical information, provides
sufficient information to establish an accurate diagnosis, the second
diagnostic procedure will become a redundant procedure. At the same time, a subsequent diagnostic
procedure can be a complementary diagnostic procedure if the first or preceding
procedures, in conjunction with clinical information, cannot provide an
accurate diagnosis. Usually, preference
of a procedure over others depends upon availability, a patient’s tolerance,
and/or the treating practitioner’s familiarity with the procedure.
4.1 IMAGING STUDIES When indicated, the following additional imaging studies can be
utilized for further evaluation of the lower extremity, based upon the
mechanism of injury, symptoms, and patient history. For specific clinical indications, see
Section 5.0, Specific Lower Extremity Injury Diagnosis, Testing, and Treatment.
The studies below are listed in frequency of use, not importance.
4.1.1 Magnetic
Resonance Imaging (MRI) are generally accepted, well-established,
and widely used diagnostic procedures. It
provides a more definitive visualization of soft tissue structures,
including ligaments, tendons, joint capsule, menisci and joint cartilage
structures, than x-ray or Computed Axial Tomography in the evaluation of
traumatic or degenerative injuries. The
addition of intravenous or intra-articular contrast can enhance definition of
selected pathologies.
4.1.1.1 The
high field, closed MRI with 1.5 or higher tesla provides better
resolution. A lower field scan may be
indicated when a patient cannot fit into a high field scanner or is too
claustrophobic despite sedation.
Inadequate resolution on the first scan may require a second MRI using a
different technique or with a reading by a musculoskeletal radiologist. All
questions in this regard should be discussed with the MRI center and/or
radiologist.
4.1.1.2 MRIs have high sensitivity and specificity
for meniscal tears and ligamentous injuries although in some cases when
physical exam findings and functional deficits indicate the need for surgery an
MRI may not be necessary. MRI is less accurate for articular cartilage defects (sensitivity 76%) than for meniscal and
ligamentous injury (sensitivity greater than 90%).
4.1.1.3 MRIs have not been shown to
be reliable for diagnosing symptomatic hip bursitis.
4.1.2 MR
Arthrography (MRA): This accepted investigation uses
the paramagnetic properties of gadolinium to shorten T1 relaxation times and
provide a more intense MRI signal. It
should be used to diagnose hip labral tears.
Pelvic MRIs are not sufficient for this purpose. Arthrograms are also useful to evaluate
mechanical pathology in knees with prior injuries and/or surgery.
4.1.3 Computed Axial Tomography (CT) is generally accepted and provides excellent visualization of bone. It
is used to further evaluate bony masses and suspected fractures not clearly
identified on radiographic window evaluation.
Instrument scatter-reduction software provides better resolution when
metallic artifact is of concern.
4.1.4 Diagnostic Sonography is
an accepted diagnostic procedure. The
performance of sonography is operator-dependent, and is best when done by a
specialist in musculoskeletal radiology or a physician appropriately trained. e. Lineal
Tomography: is infrequently used, yet may be helpful in the
evaluation of joint surfaces and bone healing.
4.1.5 Bone
Scan (Radioisotope Bone Scanning) is generally accepted,
well-established and widely used. 99MTechnecium
diphosphonate uptake reflects osteoblastic activity and may be useful in
metastatic/primary bone tumors, stress fractures, osteomyelitis, and
inflammatory lesions, but cannot distinguish between these entities.
4.1.5.1 Bone
scanning is more sensitive but less specific than MRI. It is useful for the investigation of trauma,
infection, stress fracture, occult fracture, Charcot joint, Complex Regional
Pain Syndrome and suspected neoplastic conditions of the lower extremity.
4.1.6 Other Radionuclide Scanning: Indium and gallium scans are generally
accepted, well-established, and widely used procedures usually to help diagnose
lesions seen on other diagnostic imaging studies. 67Gallium citrate scans are used
to localize tumor, infection, and abscesses.
111Indium-labeled leukocyte scanning is utilized for
localization of infection or inflammation.
4.1.7 Arthrogram
is an accepted diagnostic procedure. It may be useful in the evaluation of internal
derangement of a joint, including when MRI or other tests are contraindicated
or not available. Potential
complications of this more invasive technique include pain, infection, and
allergic reaction. Arthrography gains
additional sensitivity when combined with CT in the evaluation of internal
derangement, loose bodies, and articular cartilage surface lesions. Diagnostic arthroscopy should be considered
before arthrogram when there are strong clinical indications.
4.2 OTHER
DIAGNOSTIC TESTS:
The following diagnostic procedures listed in this subsection are listed in
alphabetical order.
4.2.1 Compartment
Pressure Testing and Measurement Devices such as pressure manometer, are useful in the
evaluation of patients who present symptoms consistent with a compartment
syndrome.
4.2.2 Diagnostic Arthroscopy (DA) allows direct visualization of the interior
of a joint, enabling the diagnosis of conditions when other diagnostic tests
have failed to reveal an accurate diagnosis; however, it should generally not
be employed for exploration purposes only.
In order to perform a diagnostic arthroscopy, the patient must have
completed at least some conservative therapy without sufficient functional
recovery per Section 5.0, Specific Lower Extremity Injury Diagnosis, Testing,
and Treatment, and meet criteria for arthroscopic repair.
4.2.2.1 DA
may also be employed in the treatment of acute joint disorders. In some cases, the mechanism of injury and
physical examination findings will strongly suggest the presence of a surgical
lesion. In those cases, it is
appropriate to proceed directly with the interventional arthroscopy.
4.2.3 Doppler Ultrasonography/Plethysmography is useful in establishing the diagnosis of
arterial and venous disease in the lower extremity and should usually be
considered prior to the more invasive venogram or arteriogram study. Doppler is less sensitive in detecting deep
vein thrombosis in the calf muscle area.
If the test is initially negative and symptoms continue, an ultrasound
should usually be repeated 7 days later to rule out popliteal thrombosis. It is also useful for the diagnosis of
popliteal mass when MRI is not available or contraindicated.
4.2.4 Electrodiagnostic
Testing Electrodiagnostic tests include, but are not
limited to Electromyography (EMG), Nerve Conduction Studies (NCS) and
Somatosensory Evoked Potentials (SSEP). These are generally accepted,
well-established and widely used diagnostic procedures. The SSEP study, although generally accepted,
has limited use. Electrodiagnostic
studies may be useful in the evaluation of patients with suspected involvement
of the neuromuscular system, including disorder of the anterior horn cell,
radiculopathies, peripheral nerve entrapments, peripheral neuropathies,
neuromuscular junction and primary muscle disease.
4.2.4.1 In
general, these diagnostic procedures are complementary to imaging procedures
such as CT, MRI, and/or myelography or diagnostic injection procedures. Electrodiagnostic studies may provide useful,
correlative neuropathophysiological information that would be otherwise
unobtainable from standard radiologic studies.
4.2.5 Personality/Psychological/Psychosocial
Interventions are generally accepted and well-established diagnostic
procedures with selective use in the acute lower extremity population, but have
more widespread use in sub-acute and chronic lower extremity populations.
4.2.5.1 Once
a diagnosis consistent with the standards of the American Psychiatric
Association Diagnostic Statistical Manual of Mental Disorders has been
determined, the patient should be evaluated for the potential need for
psychiatric medications. Use of any medication to treat a diagnosed condition
may be ordered by the authorized treating physician or by the consulting
psychiatrist. Visits for management of psychiatric medications are medical in
nature and are not a component of psychosocial treatment. Therefore, separate
visits for medication management may be necessary, depending upon the patient
and medications selected.
4.2.5.2 The
screening or diagnostic workup should have clarified and distinguished between
pre-existing, aggravated, and/or purely causative psychological conditions.
Therapeutic and diagnostic modalities include, but are not limited to,
individual counseling, and group therapy. Treatment can occur within an
individualized model, a multi-disciplinary model, or within a structured pain
management program.
4.2.5.3 A
psychologist with a Ph.D., PsyD, EdD credentials, or a Psychiatric MD/DO may
perform psychosocial treatments. Other licensed mental health providers working
in consultation with a Ph.D., PsyD, EdD, or Psychiatric MD/DO, and with
experience in treating pain management in injured workers may also perform
treatment.
4.2.5.4 Frequency:
1 to 5 times weekly for the first 4 weeks (excluding hospitalization, if
required), decreasing to 1 to 2 times per week for the second month.
Thereafter, 2 to 4 times monthly with the exception of exacerbations which may
require increased frequency of visits. Not to include visits for medication
management.
4.2.5.5 Maximum
duration: 6 to 12 months, not to include visits for medication management. For
select patients, longer supervised treatment may be required.
4.2.6 Venogram/Arteriogram: is
useful for investigation of vascular injuries or disease, including deep venous
thrombosis. Potential complications may
include pain, allergic reaction, and deep vein thrombosis.
4.3 SPECIAL
TESTS are
generally well-accepted tests and are performed as part of a skilled assessment
of the patient's capacity to return-to-work, his/her strength capacities, and
physical work demand classifications and tolerances. The procedures in this subsection are listed
in alphabetical order.
4.3.1 Computer-Enhanced
Evaluations may
include isotonic, isometric, isokinetic and/or isoinertial measurement of
movement, range of motion, balance, endurance or strength. Values obtained can include degrees of
motion, torque forces, pressures or resistance.
Indications include determining validity of effort, effectiveness of
treatment and demonstrated motivation.
These evaluations should not be used alone to determine return-to-work
restrictions.
4.3.1.1 Frequency: One time for evaluation. Can monitor improvements in strength every 3
to 4 weeks up to a total of 6 evaluations.
4.3.2 Functional Capacity Evaluation (FCE)
is a comprehensive or
modified evaluation of the various aspects of function as they relate to the
worker's ability to return-to-work.
Areas such as endurance, lifting (dynamic and static), postural tolerance,
specific range of motion, coordination and strength, worker habits,
employability, as well as psychosocial aspects of competitive employment may be
evaluated. Components of this evaluation
may include: (a) musculoskeletal screen;
(b) cardiovascular profile/aerobic capacity; (c) coordination; (d)
lift/carrying analysis; (e) job-specific activity tolerance; (f) maximum
voluntary effort; (g) pain assessment/psychological screening; and (h)
non-material and material handling activities.
An FCE may be required.
4.3.3 Jobsite Analysis is a comprehensive analysis of the physical,
mental and sensory components of a specific job. These components may include, but are not
limited to: (a) postural tolerance (static and dynamic); (b) aerobic requirements;
(c) range of motion; (d) torque/force; (e) lifting/carrying; (f) cognitive
demands; (g) social interactions; (h) visual perceptual; (i) sensation; (j)
coordination; (k) environmental requirements of a job; (l)
repetitiveness; and (m) essential job functions including job licensing
requirements. Job descriptions provided
by the employer are helpful but should not be used as a substitute for direct
observation. A Jobsite Analysis may be
required.
4.3.4 Work
Tolerance Screening (Fitness for Duty) is a determination of an individual's
tolerance for performing a specific job based on a job activity or task. A Work Tolerance Screening may be
required. The decision for performance of
a Work Tolerance Screening should be made by the therapy provider, the treating
physician, and the employer.
|
|
5.1 FOOT AND ANKLE
5.1.1 Achilles Tendonopathy or Injury and Rupture (Alternate Spelling: “Tendinopathy”):
5.1.1.1 Description/Definition: Rupture or tear
of Achilles tendon or insertional or non-insertional tendonopathy.
5.1.1.2 Occupational Relationship: Usually, tears
or ruptures are related to a fall, twisting, jumping, or sudden load on ankle
with dorsiflexion. Tendonopathy may be
exacerbated by continually walking on hard surfaces.
5.1.1.3 Specific Physical Exam Findings:
Swelling and pain at tendon, sometimes accompanied by crepitus and pain with
passive motion. Rupture or partial tear
may present with palpable deficit in tendon.
If there is a full tear, Thompson test will usually be positive. A positive Thompson's test is lack of plantar
flexion with compression of the calf when the patient is prone with the knee
flexed.
5.1.1.4 Diagnostic Testing Procedures:
Radiography may be performed to identify Haglund’s deformity; however, many
Haglund’s deformities are asymptomatic.
MRI or ultrasound may be performed if surgery is being considered for
tendonopathy or rupture.
5.1.1.5 Non-operative Treatment Procedures:
5.1.1.5.1 Initial
Treatment: Cast in non weight-bearing
for tears. Protected weight-bearing for other injuries.
5.1.1.5.2 Medications
such as analgesics and anti-inflammatories may be helpful. Refer to medication
discussions in Section 6.0 Medications and Medical Management.
5.1.1.5.3 Patient
education should include instruction in self-management techniques, ergonomics,
body mechanics, home exercise, joint protection, and weight management.
5.1.1.5.4 Benefits
may be achieved through therapeutic rehabilitation and rehabilitation
interventions. Eccentric training alone
or with specific bracing may be used for tendonopathy. Manual therapy may also be used. Therapy will
usually include range-of-motion (ROM), active therapies, and a home exercise
program. Active therapies include, proprioception training, restoring normal
joint mechanics, and clearing dysfunctions from adjacent structures. Passive as well as active therapies may be
used for control of pain and swelling. Therapy should progress to strengthening
and an independent home exercise program targeted to further improve ROM,
strength, and normal joint mechanics influenced by distal and proximal
structures. Refer to Section 6.0, Therapeutic Procedures, Non-operative.
5.1.1.5.4.1 Passive
modalities are most effective as adjunctive treatments to improve the results
of active treatment. They may be used as
found in Section 6.0, Therapeutic Procedures, Non-operative.
5.1.1.5.5 Steroid injections should generally be
avoided in these patients since this is a risk for later rupture.
5.1.1.5.6 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0. 13, Return to Work.
5.1.1.5.7 Other therapies in Section 6.0.
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.1.1.5.8 The use of PRP may be beneficial in
refractory chronic tendonopathies. Musculoskeletal
ultrasound is recommended when performing PRP.
5.1.1.6 Surgical
Indications/Considerations: Total or partial rupture.
Smoking may affect soft tissue healing through tissue hypoxia. Patients
should be strongly encouraged to stop smoking and be provided with appropriate
counseling and medication by the physician.
5.1.1.7 Operative Procedures: Repair of tendons
open or percutaneously with or without anchors may be required. Tendon grafts are used for chronic cases or
primary surgery failures when tendon tissue is poor.
5.1.1.8 Post-operative
Treatment:
5.1.1.8.1 An
individualized rehabilitation program based upon communication between the surgeon and the therapist
using therapies as outlined in Section 6.0, Therapeutic Procedures, Non-Operative.
5.1.1.8.2 Treatment
may include the following: restricted weight-bearing, bracing, active therapy
with or without passive therapy.
5.1.1.8.3 Range
of motion may begin at 3 weeks depending on wound healing. Therapy and some restrictions will usually
continue for 6 to 8 weeks.
5.1.1.8.4 Return
to work and restrictions after surgery may be made by an attending physician in
consultation with the surgeon or by the surgeon.
5.1.2 Aggravated Osteoarthritis:
5.1.2.1 Description/Definition: Internal joint
pathology of ankle.
5.1.2.1.1 Other
causative factors to consider: Prior significant injury to the ankle may
predispose the joint to osteoarthritis.
In order to entertain previous trauma as a cause, the patient should
have a medically documented injury with radiographs or MRI showing the level of
anatomic change. The prior injury should
have been at least 2 years from the presentation for the new complaints and
there should be a significant increase of pathology on the affected side in
comparison to the original imaging or operative reports and/or the opposite
un-injured extremity.
5.1.2.2 Specific Physical Exam Findings: Pain
within joint, swelling. Crepitus, locking of the joint, reduced range of
motion, pain with stress tests, angular deformities.
5.1.2.3 Diagnostic Testing Procedures: X-ray –
mechanical axis views, CT, MRI, diagnostic injection.
5.1.2.4 Non-operative Treatment Procedures:
5.1.2.4.1 Initial
Treatment: May include orthoses, custom
shoes with rocker bottom shoe inserts, and braces. Cane may also be
useful.
5.1.2.4.2 Medications
such as analgesics and anti-inflammatories may be helpful. Refer to medication
discussions in Section 6.0, Medications and Medical Management.
5.1.2.4.3 Patient
education should include instruction in self-management techniques, ergonomics,
body mechanics, home exercise, joint protection, and weight management.
5.1.2.4.4 Benefits
may be achieved through therapeutic rehabilitation and rehabilitation
interventions. They should include range-of-motion (ROM), active therapies, and
a home exercise program. Active therapies include, proprioception training,
restoring normal joint mechanics, and clearing dysfunctions from adjacent
structures. Passive as well as active
therapies may be used for control of pain and swelling. Therapy should progress
to strengthening and an independent home exercise program targeted to further
improve ROM, strength, and normal joint mechanics influenced by distal and
proximal structures. Refer to Section 6.0., Therapeutic Procedures,
Non-operative.
5.1.2.4.4.1 Passive
modalities are most effective as adjunctive treatments to improve the results
of active treatment. They may be used as found in Section 6.0., Therapeutic
Procedures, Non-operative.
5.1.2.4.5 Steroid
injections may decrease inflammation and allow the therapist to progress with
functional exercise and range of motion.
Steroid injections under significant pressure should be avoided as the
needle may be penetrating the tendon and injection into the tendon can cause
possible tendon breakdown, tendon degeneration, or rupture. Injections should be minimized for patients younger
than 30 years of age. Injections may be
performed with or without ultrasound guidance. Ultrasound guided interventional
procedure provides the ability to image soft tissues in real time and can
improve safety and accuracy of needle placement. The use of ultrasound guided procedures will
be at the discretion of the health care provider.
5.1.2.4.5.1 Time
to Produce Effect: One injection.
5.1.2.4.5.2 Maximum
Duration: 3 injections in one year spaced at least 4 to 8 weeks
apart.
5.1.2.4.5.3 Steroid
injections should be used cautiously in diabetic patients. Diabetic patients
should be reminded to check their blood glucose levels at least daily for 2
weeks after injections.
5.1.2.4.6 Return
to work with appropriate restrictions should be considered early in the course
of treatment. Refer to Section 6.0., the Return to Work subsection.
5.1.2.4.7 Other therapies in Section 6.0, Therapeutic Procedures, Non-operative
may be employed in individual cases.
5.1.2.5 Surgical
Indications/Considerations:
5.1.2.5.1 The
patient is a good surgical candidate and pain continues to interfere with ADLs
after non-surgical interventions including weight control, therapy with active
patient participation, and medication.
5.1.2.5.2 Refer
to Section 7.0 for specific indications for osteotomy, ankle fusion or
arthroplasty.
5.1.2.5.3 Implants
are less successful than similar procedures in the knee or hip. There are no quality studies comparing
arthrodesis and ankle replacement.
Patients with ankle fusions generally have good return to function and
fewer complications than those with joint replacements. Salvage procedures for ankle replacement
include revision with stemmed implant or allograft fusion. Given these factors,
an ankle arthroplasty requires prior authorization and a second opinion by a
surgeon specializing in lower extremity surgery.
5.1.2.5.4 Prior
to surgical intervention, the patient and treating physician should identify
functional operative goals and the likelihood of achieving improved ability to
perform activities of daily living or work activities and the patient should
agree to comply with the pre- and post-operative treatment plan including home
exercise. The provider should be especially careful to make sure the patient
understands the amount of post-operative therapy required and the length of
partial- and full-disability expected post-operatively.
5.1.2.5.5 In
cases where surgery is contraindicated due to obesity, it may be appropriate to
recommend a weight loss program if the patient is unsuccessful losing weight on
their own. Coverage for weight loss would continue only for motivated patients
who have demonstrated continual progress with weight loss.
5.1.2.5.6 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that carriers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
5.1.2.6 Operative Procedures: Arthroscopy, ankle arthroplasty or
fusion. Supramalleolar osteotomies can
be considered for patients with deformities or pre-existing hind foot varus or
valgus deformities.
5.1.2.7 Post-operative Treatment:
5.1.2.7.1 An
individualized rehabilitation program based upon communication between the
surgeon and the therapist using therapies as outlined in Section 6.0, Therapeutic
Procedures, Non-Operative.
5.1.2.7.2 In
all cases, communication between the physician and therapist is important to
the timing of weight-bearing and exercise progressions.
5.1.2.7.3 Treatment
may include the following: restricted weight-bearing, bracing, gait training
and other active therapy with or without passive therapy.
5.1.2.7.4 Refer
to Section 7.0 for Ankle Fusion, Osteotomy, or Arthroplasty for further
specific information.
5.1.2.7.5 Return
to work and restrictions after surgery may be made by an attending physician in
consultation with the surgeon or by the surgeon.
5.1.3 Ankle or Subtalar Joint Dislocation:
5.1.3.1 Description/Definition: Dislocation of
ankle or subtalar joint.
5.1.3.2 Occupational Relationship: Usually occurs with falling or twisting.
5.1.3.3 Specific Physical Exam Findings: Disruption of articular arrangements of
ankle, subtalar joint may be tested using ligamentous laxity tests.
5.1.3.4 Diagnostic Testing Procedures: Radiographs, CT scans. MRI may be used to assess for avascular
necrosis of the talus which may occur secondary to a dislocation.
5.1.3.5 Non-operative Treatment Procedures:
5.1.3.5.1 Initial
Treatment: Closed reduction under anesthesia
with pre- and post-reduction neurovascular assessment followed by casting and
weight-bearing limitations.
5.1.3.5.2 Medications
such as analgesics and anti-inflammatories may be helpful. Refer to medication
discussions in Section 6.0, Medications and Medical Management.
5.1.3.5.3 Patient
education should include instruction in self-management techniques, ergonomics,
body mechanics, home exercise, joint protection, and weight management.
5.1.3.5.4 Benefits
may be achieved through therapeutic rehabilitation and rehabilitation
interventions. They should include range of motion (ROM), active therapies, and
a home exercise program. Active therapies include, proprioception training,
restoring normal joint mechanics, and clearing dysfunctions from adjacent
structures. Passive as well as active
therapies may be used for control of pain and swelling. Therapy should progress
to strengthening and an independent home exercise program targeted to further
improve ROM, strength, and normal joint mechanics influenced by distal and
proximal structures. Refer to Section 6.0, Therapeutic Procedures,
Non-operative.
5.1.3.5.4.1 Passive
modalities are most effective as adjunctive treatments to improve the results
of active treatment. They may be used as
found in Section 6.0, Therapeutic Procedures, Non-operative.
5.1.3.5.5 Return
to work with appropriate restrictions should be considered early in the course
of treatment. Refer to Section 6.0, Return to Work.
5.1.3.5.6 Other
therapies in Section 6.0, Therapeutic Procedures, Non-operative may be employed
in individual cases.
5.1.3.6 Surgical Indications/Considerations:
Inability to reduce closed fracture, association with unstable fractures.
5.1.3.7 Operative Procedures: Open or closed reduction of dislocation.
5.1.3.8 Post-operative Treatment:
5.1.3.8.1 An
individualized rehabilitation program based upon communication between the
surgeon and the therapist using therapies as outlined in Section 6.0,
Therapeutic Procedures, Non-Operative.
5.1.3.8.2 Treatment
usually includes initial immobilization with restricted weight-bearing,
followed by bracing and active therapy with or without passive therapy.
5.1.3.8.3 Return
to work and restrictions after surgery may be made by an attending physician in
consultation with the surgeon or by the surgeon.
5.1.4 Ankle Sprain/Fracture:
5.1.4.1 Description/Definition: An injury to
the ankle joint due to abnormal motion of the talus that causes a stress on the
malleolus and the ligaments. Injured ligaments in order of disruption include
the anterior talofibular ligament (ATFL), calcaneofibular ligament (CFL),
posterior talofibular ligament (PTFL), deltoid ligaments, and syndesmotic
ligaments. Instability can result from a fracture of a malleolus (malleolli),
rupture of ligaments, or a combination. Circumstances surrounding the injury,
including consideration of location and additional injuries are of importance.
Additionally, the position of the foot at the time of injury is helpful in
determining the extent and type of injury. Grading of soft tissue injuries
includes:
5.1.4.1.1 Grade
1 Injury: those with overstretching or microscopic tears of the ligament,
minimal swelling, normal stress testing, and the ability to bear weight.
5.1.4.1.2 Grade
2 Injury: have partial disruption of the ligament, significant swelling,
indeterminate results on stress testing, and difficulty bearing weight.
5.1.4.1.3 Grade
3 Injury: have a ruptured ligament, swelling and ecchymosis, abnormal results
on stress testing, and the inability to bear weight. May also include a chip
avulsion fracture on x-ray.
5.1.4.2 Occupational Relationship: Usually
occurs from sudden twisting, direct blunt trauma and falls. Inversion of the
ankle with a plantar-flexed foot is the most common mechanism of injury.
5.1.4.3 Specific Physical Exam Findings: varies
with individual. With lower grade sprains the ankle may be normal appearing
with minimal tenderness on examination. The ability/inability to bear weight,
pain, swelling, or ecchymosis should be noted. If the patient is able to
transfer weight from one foot onto the affected foot and has normal physical
findings, then likelihood of fracture is reduced. Stress testing using the anterior drawer
stress test, the talar tilt test and the external rotation stress test may be
normal or abnormal depending on the involved ligament.
5.1.4.3.1 Syndesmotic
injury can occur with external rotation injuries and requires additional
treatment. Specific physical exam tests include the squeeze test and external
rotation at neutral.
5.1.4.4 Diagnostic Testing Procedures:
Radiographs. Refer to Initial Diagnostic Section which generally follows the
Ottawa Ankle Rules. The Ottawa Ankle Rules are a decision aid for radiography.
Commonly missed conditions include ankle syndesmosis or fractures. The
instrument has a sensitivity of almost 100% and a modest specificity, and its
use should reduce the number of unnecessary radiographs by 30 to 40%.
5.1.4.4.1 For
an acute, unstable ankle or a repeat or chronic ankle injury, a MRI and/or
diagnostic injection may be ordered.
Arthroscopy can be used in unusual cases with persistent functional
instability and giving way of the ankle, after conservative treatment, to
directly visualize the ruptured ligament(s).
5.1.4.5 Non-operative Treatment Procedures:
5.1.4.5.1 Initial
treatment for patients able to bear weight:
NSAIDs, RICE (rest, ice, compression and elevation), and early
functional bracing is used. In addition,
crutches may be beneficial for comfort. Early functional treatment including
range of motion and strengthening exercises along with limited weight-bearing,
are preferable to strict immobilization with rigid casting for improving
outcome and reducing time to return to work.
5.1.4.5.2 Initial
treatment for patients unable to bear weight: bracing plus NSAIDs and RICE are
used. When patient becomes able to bear weight a walker boot is frequently
employed. There is no clear evidence
favoring ten days of casting over pneumatic bracing as initial treatment for
patients who cannot bear weight three days post injury. There is good evidence that use of either
device combined with functional therapy results in similar long-term recovery.
5.1.4.5.2.1 There
is some evidence that functional rehabilitation has results superior to six
weeks of immobilization.
5.1.4.5.2.2 Small
avulsion fractures of the fibula with minimal or no displacement can be treated
as an ankle sprain.
5.1.4.5.2.3 For
patients with a clearly unstable joint, immobilize with a short leg plaster
cast or splint for 2 to 6 weeks along with early weight-bearing.
5.1.4.5.3 Balance/coordination
training is a well-established treatment which improves proprioception and may
decrease incidence of recurrent sprains.
5.1.4.5.4 Medications
such as analgesics and anti-inflammatories may be helpful. Refer to medication
discussions in Section 6.0, Medications and Medical Management.
5.1.4.5.5 Patient
education should include instruction in self-management techniques, ergonomics,
body mechanics, home exercise, joint protection, and weight management.
5.1.4.5.6 Heel
wedges or other orthotics may be used for rear foot varus or valgus
deformities.
5.1.4.5.6.1 There
is good evidence that semi-rigid orthoses or pneumatic braces prevent ankle
sprains during high risk physical activities and they should be used as
appropriate after acute sprains.
5.1.4.5.7 When
fractures are involved refer to comments related to osteoporosis in Section
6.0, Therapeutic Procedures, Non-operative,
subsection, Osteoporosis Management.
5.1.4.5.8 Smoking may affect fracture healing.
Patients should be strongly encouraged to stop smoking and be provided with
appropriate counseling by the physician.
5.1.4.5.9 Return-to-work
with appropriate restrictions should be considered early in the course of treatment.
Refer to Section 6.0, Return to Work.
5.1.4.5.10 Other
therapies in Section 6.0, Therapeutic Procedures, Non-operative, including
manual therapy may be employed in individual cases.
5.1.4.5.11 Hyperbaric
oxygen therapy is not recommended.
5.1.4.6 Surgical
Indications/Considerations:
5.1.4.6.1 Acute
surgical indications include sprains with displaced fractures, syndesmotic
disruption or ligament sprain associated with a fracture causing
instability.
5.1.4.6.2 There
is no conclusive evidence that surgery as opposed to functional treatment for
an uncomplicated Grade I-III ankle sprain improves patient outcome.
5.1.4.6.3 Chronic
indications are functional problems, such as recurrent instability, remaining
after at least 2 months of appropriate therapy including active participation
in a non-operative therapy program including balance training.
5.1.4.6.4 Prior
to surgical intervention, the patient and treating physician should identify
functional operative goals and the likelihood of achieving improved ability to
perform activities of daily living or work activities and the patient should
agree to comply with the pre- and post-operative treatment plan including home
exercise. The provider should be especially careful to make sure the patient
understands the amount of post-operative therapy required and the length of
partial- and full-disability expected post-operatively.
5.1.4.6.5 If injury is a sprain: Smoking may
affect soft tissue healing through tissue hypoxia. Patients should be strongly
encouraged to stop smoking and be provided with appropriate counseling by the
physician.
5.1.4.6.6 If
injury is a fracture: Because smokers have a higher risk of non-union and
post-operative costs, it is recommended that carriers cover a smoking cessation
program peri-operatively. Physicians may
monitor smoking cessation with laboratory tests such as cotinine levels for
long-term cessation.
5.1.4.7 Operative Treatment: Repair of
fractures or other acute pathology as necessary. Primary ligament ankle
reconstruction with possible tendon transplant.
5.1.4.8 Post-operative Treatment:
5.1.4.8.1 An
individualized rehabilitation program based upon communication between the
surgeon and the therapist using therapies as outlined in Section 6.0,
Therapeutic Procedures, Non-Operative.
Treatment may include short-term post surgical casting. In all cases,
communication between the physician and therapist is important to the timing of
weight-bearing and exercise progressions.
5.1.4.8.1.1 There
is some evidence that more rapid recovery occurs with functional rehabilitation
compared to six weeks of immobilization in a cast.
5.1.4.8.2 The
surgical procedures and the patient’s individual results dictate the amount of
time a patient has non weight-bearing restrictions. Fractures usually require 6 to 8 weeks while
tendon transfers may be 6 weeks. Other soft tissue repairs, such as the
Brostrom lateral ankle stabilization, may be as short as 3 weeks.
5.1.4.8.3 Return
to work and restrictions after surgery may be made by an attending physician in
consultation with the surgeon or by the surgeon.
5.1.5 Calcaneal Fracture:
5.1.5.1 Description/Definition:
Osseous fragmentation/separation confirmed by diagnostic studies.
5.1.5.2 Occupational Relationship: Usually occurs by fall or crush injury.
5.1.5.3 Specific
Physical Exam Findings: Pain with
range of motion and palpation of calcaneus.
Inability to bear weight, mal-positioning of heel, possible impingement
of sural nerve.
5.1.5.4 Diagnostic
Testing Procedures: Radiographs and
CT scan to assess for intra-articular involvement. Lumbar films and urinalysis are usually
performed to rule out lumbar crush fractures when the mechanism of injury is a
fall from a height.
5.1.5.5 Non-operative Treatment Procedures:
5.1.5.5.1 Initial Treatment: Non weight-bearing 6 to 8 weeks, followed by weight-bearing cast at physician’s
discretion and active therapy with
or without passive therapy.
5.1.5.5.2 Medications
such as analgesics and anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0, the Medications
and Medical Management subsection.
5.1.5.5.3 Patient
education should include instruction in self-management techniques, ergonomics,
body mechanics, home exercise, joint protection, and weight management.
5.1.5.5.4 Refer
to comments related to osteoporosis in Section 6.0, Therapeutic Procedures,
Non-operative, Osteoporosis Management.
5.1.5.5.5 Smoking
may affect fracture healing. Patients should be strongly encouraged to stop
smoking and be provided with appropriate counseling by the physician.
5.1.5.5.6 Return
to work with appropriate restrictions should be considered early in the course
of treatment. Refer to Section 6.0, Return to Work.
5.1.5.5.7 Other
therapies in Section 6.0, Therapeutic Procedures, Non-operative may be employed
in individual cases.
5.1.5.6 Surgical
Indications/Considerations: Displacement of fragments, joint depression,
intra-articular involvement, mal-position of heel. Sanders Types II and III are
generally repaired surgically. However, the need for surgery will depend on the
individual case. Relative
contraindications: smoking, diabetes, or
immunosuppressive disease.
5.1.5.6.1 Because
smokers have a higher risk of non-union and post-operative costs, it is
recommended that carriers cover a smoking cessation program
peri-operatively. Physicians may
monitor smoking cessation with laboratory tests such as cotinine levels for
long-term cessation.
5.1.5.7 Operative Procedures: Open reduction
internal fixation. Subtalar fusion may be necessary in some cases when the
calcaneus is extremely comminuted.
External fixation has been used when the skin condition is poor.
5.1.5.7.1 Complications
may include wound infections requiring skin graft.
5.1.5.8 Post-operative Treatment:
5.1.5.8.1 An
individualized rehabilitation program based upon communication between the
surgeon and the therapist using the therapies as outlined in Section 6.0,
Therapeutic Procedures, Non-Operative.
In all cases, communication between the physician and therapist is
important to the timing of weight-bearing and exercise progressions.
5.1.5.8.2 The
patient is usually non weight-bearing for 6 to 8 weeks followed by
weight-bearing for approximately 6 to 8 weeks at physician’s discretion.
5.1.5.8.3 Treatment
may include the following: restricted weight-bearing, bracing, active therapy
with or without passive therapy.
5.1.5.8.4 Return
to work and restrictions after surgery may be made by an attending physician in
consultation with the surgeon or by the surgeon.
5.1.6 Chondral and Osteochondral Defects:
5.1.6.1 Description/Definition:
Cartilage or cartilage and bone defect of the talar surface. May be associated
with ankle sprain or other injuries.
5.1.6.2 Occupational Relationship:
Usually caused by a traumatic ankle injury.
5.1.6.3 Specific Physical Exam Findings:
Ankle effusion, pain in joint and with walking.
5.1.6.4 Diagnostic
Testing Procedures: MRI may show
bone bruising, osteochondral lesion, or possibly articular cartilage
injury. Radiographs, contrast
radiography, CT may also be used.
5.1.6.5 Non-operative Treatment Procedures:
5.1.6.5.1 Initial
Treatment: Acute injuries may require immobilization followed by active therapy
with or without passive therapy.
5.1.6.5.2 Medications
such as analgesics and anti-inflammatories may be helpful. Refer to medication
discussions in Section 6.0, Medications and Medical Management.
5.1.6.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.1.6.5.4 Benefits
may be achieved through therapeutic rehabilitation and rehabilitation
interventions. They should include range-of-motion (ROM), active therapies, and
a home exercise program. Active therapies include, proprioception training,
restoring normal joint mechanics, and clearing dysfunctions from adjacent
structures. Passive as well as active
therapies may be used for control of pain and swelling. Therapy should progress
to strengthening and an independent home exercise program targeted to further
improve ROM, strength, and normal joint mechanics influenced by distal and
proximal structures. Refer to Section 6.0,
Therapeutic Procedures, Non-operative.
5.1.6.5.4.1 Passive
modalities are most effective as adjunctive treatments to improve the results
of active treatment. They may be used as
found in Section 6.0, Therapeutic Procedures, Non-operative.
5.1.6.5.5 Return
to work with appropriate restrictions should be considered early in the course
of treatment. Refer to Section 6.0, Return to Work.
5.1.6.5.6 Other
therapies in Section 6.0, Therapeutic Procedures, Non-operative may be employed
in individual cases.
5.1.6.6 Surgical Indications/Considerations:
5.1.6.6.1 Functional
deficits not responsive to conservative therapy. Identification of an osteochondral lesion by
diagnostic testing procedures should be done to determine the size of the
lesion and stability of the joint.
5.1.6.6.2 Microfracture
is the initial treatment unless there are other anatomic variants such as a
cyst under the bone.
5.1.6.6.3 Osteochondral
Autograft Transfer System (OATS) may be effective in patients without other
areas of osteoarthritis, a BMI of less than 35 and a failed microfracture. This procedure may be indicated when
functional deficits interfere with activities of daily living and/or job
duties 6 to 12 weeks after a failed
microfracture with active patient participation in non-operative therapy. This procedure is only appropriate in a small
subset of patients and requires prior authorization.
5.1.6.6.4 Autologous
cartilage cell implant is not FDA approved for the ankle and therefore not
recommended.
5.1.6.6.5 Prior
to surgical intervention, the patient and treating physician should identify
functional operative goals and the likelihood of achieving improved ability to
perform activities of daily living or work activities and the patient should
agree to comply with the pre- and post-operative treatment plan including home
exercise. The provider should be especially careful to make sure the patient
understands the amount of post-operative therapy required and the length of
partial- and full-disability expected post-operatively.
5.1.6.6.6 Smoking
may affect tissue healing through tissue hypoxia. Patients should be strongly encouraged to stop smoking and
be provided with appropriate counseling
by the physician.
5.1.6.7 Operative
Procedures: Arthroscopy with debridement or shaving
of cartilage, microfracture, mosiacplasty, fixation of loose osteochondral
fragments.
5.1.6.8 Post-operative Treatment:
5.1.6.8.1 An
individualized rehabilitation program based upon communication between the surgeon and the therapist
and using therapies as outlined in Section 6.0, Therapeutic Procedures, Non-Operative. In all cases, communication between the
physician and therapist is important to the timing of weight-bearing and
exercise progressions.
5.1.6.8.2 Treatment
may include the following: restricted weight-bearing, bracing, active therapy
with or without passive therapy.
5.1.6.8.3 Return
to work and restrictions after surgery may be made by an attending physician in
consultation with the surgeon or by the surgeon.
5.1.7 Heel Spur Syndrome/Plantar Fasciitis:
5.1.7.1 Description:
Pain along the inferior aspect of the heel at the calcaneal attachment of the
plantar fascia and/or along the course of the plantar fascia.
5.1.7.2 Occupational
Relationship: Usually, the condition may be exacerbated by prolonged
standing or walking on hard surfaces.
Acute injury may be caused by trauma.
This may include jumping from a height or hyperextension of the forefoot
upon the rear foot.
5.1.7.3 Specific
Physical Exam Findings: Pain with palpation at the inferior attachment of
the plantar fascia to the os calcis may be associated with calcaneal spur. Gastrocnemius tightness may be tested with
the Silfverskiöld test. The foot is
dorsiflexed with the knee extended and then with the knee flexed. The test for gastrocnemius tightness is
considered positive if dorsiflexion is greater with the knee flexed than with
the knee extended.
5.1.7.4 Diagnostic
Testing Procedures: Standard
radiographs to rule out fracture, identify spur after conservative
therapy. Bone scans and/or MRI may be
used to rule out stress fractures in chronic cases.
5.1.7.5 Non-operative Treatment Procedures:
5.1.7.5.1 Initial
Treatment: This condition usually
responds to conservative management consisting of eccentric exercise of the
gastrocnemius, plantar fascial stretching, taping, soft-tissue mobilization,
night splints, and orthotics. Therapy
may include passive therapy, taping, and injection therapy.
5.1.7.5.2 Shock
absorbing shoe inserts may prevent back and lower extremity problems in some
work settings.
5.1.7.5.3 Medications
such as analgesics and anti-inflammatories may be helpful. Refer to medication
discussions in Section 6.0, Medications and Medical Management.
5.1.7.5.4 Patient
education should include instruction in self-management techniques, ergonomics,
body mechanics, home exercise, joint protection, and weight management.
5.1.7.5.5 Steroid
injections may decrease inflammation and allow the therapist to progress with
functional exercise and range of motion.
Steroid injections under significant pressure should be avoided as the
needle may be penetrating the tendon and injection into the tendon can cause
possible tendon breakdown, tendon degeneration, or rupture. Injections should be minimized for patients younger
than 30 years of age. Injections may be
performed with or without ultrasound guidance. Ultrasound guided interventional
procedure provides the ability to image soft tissues in real time and can
improve safety and accuracy of needle placement. The use of ultrasound guided procedures will
be at the discretion of the health care provider.
5.1.7.5.5.1 Time
to Produce Effect: One injection.
5.1.7.5.5.2 Maximum
Duration: 3 injections in one year spaced at least 4 to 8 weeks apart.
5.1.7.5.5.3 Steroid injections should be used
cautiously in diabetic patients. Diabetic patients should be reminded to check
their blood glucose levels at least daily for 2 weeks after injections.
5.1.7.5.6 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.1.7.5.7 After
four months of failed therapy, Extracorporeal Shock Wave Therapy (ESWT) trial
may be considered prior to surgery.
Refer to Section 6.0, Therapeutic Procedures, Non-operative.
5.1.7.5.8 Other
therapies in Section 6.0,Therapeutic Procedures, Non-operative may be employed
in individual cases.
5.1.7.6 Surgical Indications/Considerations:
5.1.7.6.1 Surgery
is employed only after failure of at least 4 to 6 months of active patient
participation in non-operative treatment.
5.1.7.6.2 Indications
for a gastrocnemius recession include a positive Silfverskiöld test. This procedure does not weaken the arch as
may occur with a plantar fascial procedure, however, there is a paucity of
literature on this procedure.
5.1.7.6.3 Prior
to surgical intervention, the patient and treating physician should identify
functional operative goals and the likelihood of achieving improved ability to
perform activities of daily living or work activities and the patient should
agree to comply with the pre- and post-operative treatment plan including home
exercise. The provider should be especially careful to make sure the patient
understands the amount of post-operative therapy required and the length of
partial- and full-disability expected post-operatively.
5.1.7.6.4 Smoking
may affect soft tissue healing through tissue hypoxia. Patients should be
strongly encouraged to stop smoking and be provided with appropriate counseling
by the physician.
5.1.7.7 Operative
Treatment Procedures: Plantar
fascial release with or without calcaneal spur removal, endoscopic or open
gastrocnemius recession.
5.1.7.8 Post-operative Treatment:
5.1.7.8.1 An
individualized rehabilitation program based upon communication between the
surgeon and the therapist using therapies as outlined in Section 6.0,
Therapeutic Procedures, Non-Operative.
5.1.7.8.2 Treatment
may include the following: restricted weight-bearing, bracing, active therapy
with or without passive therapy. Usually non weight-bearing for 7 to 10 days
followed by weight-bearing cast or shoe for four weeks; however, depending on
the procedure some patients may be restricted from weight-bearing for 4 to 6
weeks.
5.1.7.8.3 Return
to work and restrictions after surgery may be made by an attending physician in
consultation with the surgeon or by the surgeon.
5.1.8 Metatarsal-Phalangeal, Tarsal-Metatarsal
and Interphalangeal Joint Arthropathy:
5.1.8.1 Description/Definition: Internal
derangement of joint.
5.1.8.2 Occupational Relationship: Usually from
jamming, contusion, crush injury, repetitive impact, or post-traumatic
arthrosis.
5.1.8.3 Specific Physical Exam Findings: Pain with palpation and ROM of joint,
effusion. The piano key test may be used, where the examiner stabilizes the
heel with one hand and presses down on the distal head of the metatarsals,
assessing for pain proximally.
5.1.8.4 Diagnostic Testing Procedures: Radiographs, diagnostic joint injection, CT,
MRI.
5.1.8.5 Non-operative Treatment Procedures:
5.1.8.5.1 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section
6.0, Medications and Medical Management.
5.1.8.5.2 Patient
education should include instruction in self-management techniques, ergonomics,
body mechanics, home exercise, joint protection, and weight management.
5.1.8.5.3 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. They should
include range-of-motion (ROM), active therapies, and a home exercise program.
Active therapies include, proprioception training, restoring normal joint
mechanics, and clearing dysfunctions from adjacent structures. Passive as well as active therapies may be
used for control of pain and swelling. Orthotics and iontophoresis are usually
included. A carbon fiber Morton extension may be useful. Therapy should
progress to strengthening and an independent home exercise program targeted to
further improve ROM, strength, and normal joint mechanics influenced by distal
and proximal structures. Refer to
Section 6.0, Therapeutic Procedures, Non-operative.
5.1.8.5.3.1 Passive
modalities are most effective as adjunctive treatments to improve the results
of active treatment. They may be used as
found in Section 6.0, Therapeutic Procedures, Non-operative.
5.1.8.5.4 Steroid injections may decrease
inflammation and allow the therapist to progress with functional exercise and
range of motion. Steroid injections
under significant pressure should be avoided as the needle may be penetrating
the tendon and injection into the tendon can cause possible tendon breakdown,
tendon degeneration, or rupture.
Injections should be minimized for patients younger than 30 years of
age.
5.1.8.5.4.1 Time to Produce Effect: One
injection.
5.1.8.5.4.2 Maximum Duration: 3 injections in
one year spaced at least 4 to 8 weeks apart.
5.1.8.5.4.3 Steroid injections should be used
cautiously in diabetic patients. Diabetic patients should be reminded to check
their blood glucose levels at least daily for 2 weeks after injections.
5.1.8.5.5 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.1.8.5.6 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.1.8.6 Surgical Indications/Considerations:
5.1.8.6.1 Pain, unresponsive to conservative
care and interfering with activities of daily living.
5.1.8.6.2 First metatarsal
arthritis or avascular necrosis can interfere with function and gait.
5.1.8.6.3 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.1.8.6.4 Smoking may affect soft tissue
healing through tissue hypoxia. Patients should be strongly encouraged to stop
smoking and be provided with appropriate counseling by the physician.
5.1.8.7 Operative
Procedures: If debridement of the
arthritic joint and other conservative treatment is unsuccessful in correcting
gait and walking tolerance, other procedures may be considered. Other procedures include: fusion of first
metatarsal-phalangeal joint, chilectomy, osteotomies, Keller arthroplasty and
soft tissue procedures.
5.1.8.7.1 There
is some evidence that the first metatarsal-phalangeal
joint arthritis is better treated with arthrodesis than arthroplasty for pain
and functional improvement.
Therefore, total joint arthroplasties are not recommended for any
metatarsal-phalangeal joints due to less successful outcomes than fusions. There may be an exception for first and
second metatarsal-phalangeal joint arthroplasties when a patient is older than
60, has low activity levels, and cannot tolerate non weight-bearing for
prolonged periods or is at high risk for non-union.
5.1.8.7.2 Metallic
hemi-arthroplasties are still considered experimental as long-term outcomes
remain unknown in comparison to arthrodesis, and there is a significant
incidence of subsidence. Therefore,
these are not recommended at this time.
5.1.8.8 Post-operative Treatment:
5.1.8.8.1 An individualized rehabilitation
program based upon communication between
the surgeon and the therapist using therapies as outlined in Section 6.0,
Therapeutic Procedures, Non-Operative.
In all cases, communication between the physician and therapist is
important to the timing of weight-bearing and exercise progressions.
5.1.8.8.2 For fusions and osteotomies, reduced
weight-bearing and the use of special shoes will be necessary for at least 6
weeks post operative. For other procedures early range-of-motion, bracing,
and/or orthotics. Treatment usually also includes other active therapy with or
without passive therapy.
5.1.8.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.1.9 Midfoot (Lisfranc) Fracture/Dislocation:
5.1.9.1 Description/Definition:
Fracture/ligamentous disruption of the tarsal-metatarsal joints, i.e.,
metatarsal-cuneiform and metatarsal-cuboid bones.
5.1.9.2 Occupational Relationship: Usually occurs from a fall, crush, axial load
with a plantar flexed foot, or abductory force on the forefoot.
5.1.9.3 Specific Physical Exam Findings: Pain and swelling at the Lisfranc joint,
first and/or second metatarsal cuneiform articulation, palpable dorsal
dislocation, pain on forced abduction.
5.1.9.3.1 Dislocation may not always be
apparent. Pronation and supination of the forefoot with the calcaneus fixed in
the examiners opposite hand may elicit pain in a Lisfranc injury,
distinguishing it from an ankle sprain, in which this maneuver is expected to
be painless. The piano key test may be used, where the examiner stabilizes the
heel with one hand and presses down on the distal head of the metatarsal,
assessing for pain proximally. The dorsalis pedis artery crosses the second
metatarsal and may be disrupted. Therefore, the dorsalis pedis pulse and
capillary filling should be assessed.
5.1.9.4 Diagnostic Testing Procedures: X-rays, CT scans, MRI, mid-foot stress
x-rays.
5.1.9.5 Non-operative Treatment Procedures:
5.1.9.5.1 Initial Treatment: If minimal or no displacement then casting,
non weight-bearing 6 to 8 weeks. Orthoses may be used later.
5.1.9.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
Medications and Medical Management.
5.1.9.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.1.9.5.4 Refer to comments related to
osteoporosis in Section 6.0, Therapeutic Procedures, Non-operative,
Osteoporosis Management.
5.1.9.5.5 Smoking may affect fracture healing.
Patients should be strongly encouraged to stop smoking and be provided with
appropriate counseling by the physician.
5.1.9.5.6 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.1.9.5.7 Other therapies in Section 6.0, Therapeutic
Procedures, Non-operative may be employed in individual cases.
5.1.9.6 Surgical
Indications/Considerations:
Displacement of fragments or intra-articular fracture. Most Lisfranc fracture/dislocations are
treated surgically.
5.1.9.6.1 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that carriers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
5.1.9.7 Operative
Procedures: Open reduction internal
fixation with possible removal of hardware at approximately 3 to 6 months,
pending healing status. Alternatively,
arthrodesis of the medial 2 or 3 metatarsals.
5.1.9.8 Post-operative Treatment:
5.1.9.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist using
treatments as outlined in Section 6.0, Therapeutic Procedures, Non-Operative. In all cases, communication between the
physician and therapist is important to the timing of weight-bearing and
exercise progressions.
5.1.9.8.2 The patient is usually in cast or
fracture walker for 6 to 8 weeks non weight-bearing. Orthoses may be indicated after healing.
5.1.9.8.3 Treatment may include the following:
restricted weight-bearing, bracing, active therapy with or without passive
therapy.
5.1.9.8.4 Return
to work and restrictions after surgery may be made by an attending physician in
consultation with the surgeon or by the surgeon.
5.1.10 Morton’s Neuroma:
5.1.10.1 Description: This condition is a perineural fibrosis of
the intermetatarsal nerve creating pain and/or paresthesias in the forefoot
region. Symptoms appear with
weight-bearing activities. Usually
occurs between the third and fourth metatarsals or between the second and third
metatarsals.
5.1.10.2 Occupational Relationship: Acute injuries may include excessive loading
of the forefoot region caused from jumping or pushing down on the ball of the
foot. Non-traumatic occurrences are determined at physician’s discretion after
review of environmental and biomechanical risk factors.
5.1.10.3 Specific Physical Exam Findings:
Paresthesias and/or pain with palpation of the inter-metatarsal nerve.
Mulder’s sign, a palpable click from compression of the nerve, or Tinel’s sign.
5.1.10.4 Diagnostic Testing Procedures: Radiographs to rule out osseous
involvement. Diagnostic and therapeutic
injections. Diagnosis is usually based
on clinical judgment; however, MRI and ultrasound imaging have also been
employed in difficult cases.
5.1.10.5 Non-operative Treatment Procedures:
5.1.10.5.1 Initial Treatment: Nonsteroidal
anti-inflammatories and foot orthoses are primary treatments.
5.1.10.5.2 Medications such as analgesics and
anti-inflammatories are usually helpful.
Refer to medication discussions in Section 6.0, Medications and Medical
Management.
5.1.10.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.1.10.5.4 Steroid injections may decrease
inflammation and allow the therapist to progress with functional exercise and
range of motion. Steroid injections
under significant pressure should be avoided as the needle may be penetrating
the tendon and injection into the tendon can cause possible tendon breakdown,
tendon degeneration, or rupture.
Injections should be minimized for patients younger than 30 years of
age.
5.1.10.5.4.1 Time to Produce Effect: One
injection.
5.1.10.5.4.2 Maximum Duration: 3 injections in
one year spaced at least 4 to 8 weeks apart.
5.1.10.5.4.3 Steroid injections should be used
cautiously in diabetic patients. Diabetic patients should be reminded to check
their blood glucose levels at least daily for 2 weeks after injections.
5.1.10.5.5 Alcohol injections are thought to
produce a chemical neurolysis. Alcohol
injection with ultrasound guidance may be used to decrease symptoms.
5.1.10.5.5.1 Optimum Duration: 4 treatments.
5.1.10.5.5.2 Maximum Duration: 7 treatments.
5.1.10.5.6 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.1.10.5.7 Other therapies in Section 6.0,Therapeutic
Procedures, Non-operative may be employed in individual cases.
5.1.10.6 Surgical Indications/Considerations:
5.1.10.6.1 Functional deficits
persisting after 2 to 3 months of active participation in therapy.
5.1.10.6.2 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.1.10.6.3 Smoking may affect soft tissue healing
through tissue hypoxia. Patients should be strongly encouraged to stop smoking
and be provided with appropriate counseling by the physician.
5.1.10.7 Operative Procedures: Excision of the neuroma; nerve transection or
transposition.
5.1.10.8 Post-operative Treatment:
5.1.10.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist using
therapies as outlined in Section 6.0, Therapeutic Procedures, Non-Operative.
5.1.10.8.2 Treatment may involve a period of non
weight-bearing for up to two weeks, followed by gradual protected
weight-bearing 4 to 6 weeks.
5.1.10.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.1.11 Pilon Fracture:
5.1.11.1 Description/Definition:
Crush/comminution fracture of distal metaphyseal tibia that has intra-articular
extensions into the weight-bearing surface of the tibio-talar joint.
5.1.11.2 Occupational Relationship: Usually from a fall.
5.1.11.3 Specific Physical Exam Findings: Swelling, pain with weight-bearing, ecchymosis,
and palpable tenderness.
5.1.11.4 Diagnostic Testing Procedures: Radiographs, CT scans.
5.1.11.5 Non-operative Treatment Procedures:
5.1.11.5.1 Initial Treatment: Prolonged non weight-bearing at physician’s
discretion.
5.1.11.5.2 Medications such as analgesics and
anti-inflammatories may be helpful.
Refer to medication discussions in Section 6.0, Medications and Medical
Management.
5.1.11.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.1.11.5.4 Refer to comments related to
osteoporosis in Section 6.0, Therapeutic Procedures, Non-operative,
Osteoporosis Management.
5.1.11.5.5 Smoking may affect fracture healing.
Patients should be strongly encouraged to stop smoking and be provided with
appropriate counseling by the physician.
5.1.11.5.6 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.1.11.5.7 Other therapies in Section 6.0, Therapeutic
Procedures, Non-operative may be employed in individual cases.
5.1.11.6 Surgical Indications/Considerations: Displacement of fracture, severe comminution
necessitating primary fusion.
5.1.11.6.1 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that insurers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
5.1.11.7 Operative Procedures: Open reduction internal fixation, fusion,
external fixation. In some cases staged
procedures may be necessary beginning with external fixation.
5.1.11.8 Post-operative Treatment:
5.1.11.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist using
treatment as outlined in Section 6.0, Therapeutic Procedures, Non-Operative. In all cases, communication between the
physician and therapist is important to the timing of weight-bearing and
exercise progressions.
5.1.11.8.2 Treatment
may include the following: restricted weight-bearing, bracing, active therapy
with or without passive therapy.
5.1.11.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.1.12 Posterior Tibial Tendon Dysfunction:
5.1.12.1 Description/Definition:
Pain in the posteromedial ankle with plantar flexion.
5.1.12.2 Occupational Relationship: Usually from repetitive or forced
plantar flexion after an ankle sprain or athletic activity.
5.1.12.3 Specific Physical Exam
Findings: Painful posterior tibial
tendon with active and passive non weight-bearing motion, reproduction of pain
with forced plantar flexion and inversion of the ankle, difficulty performing
single heel raise, pain with palpation from the posterior medial foot along the
medial malleous to the navicular greater tuberosity. The patient should also be evaluated for a
possible weak gluteus medius as a contributing factor.
5.1.12.4 Diagnostic Testing Procedures:
X-ray, MRI may be used to rule out other diagnoses.
5.1.12.5 Non-operative Treatment Procedures:
5.1.12.5.1 Initial Treatment: Short ankle articulated orthosis and therapy
including low-load strengthening exercises with progression to home
program. Other active and passive
therapy including iontophoresis, orthotics and possible strengthening for the
gluteus medius.
5.1.12.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
Medications and Medical Management.
5.1.12.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.1.12.5.4 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.1.12.5.5 Other
therapies in Section 6.0, Therapeutic Procedures, Non-operative may be employed
in individual cases.
5.1.12.6 Surgical Indications/Considerations:
5.1.12.6.1 Failure of non-operative
treatment. Surgery is rarely necessary
as success rate for non-operative treatment is around 90%.
5.1.12.6.2 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.1.12.6.3 Smoking may affect soft tissue healing
through tissue hypoxia. Patients should be strongly encouraged to stop smoking
and be provided with appropriate counseling by the physician.
5.1.12.7 Operative Procedures:
Resection of anomolous muscle segments or tenolysis. In severe cases, tendon transfer, osteotomies
and/or arthrodesis may be necessary.
5.1.12.8 Post-operative Treatment:
5.1.12.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist and
using therapies as outlined in Section 6.0, Therapeutic Procedures, Non-Operative.
5.1.12.8.2 Treatment may include the following:
restricted weight-bearing, bracing, active therapy with or without passive
therapy.
5.1.12.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.1.13 Puncture Wounds of the Foot:
5.1.13.1 Description/Definition: Penetration of skin by foreign object.
5.1.13.2 Occupational Relationship:
Usually by stepping on foreign object, open wound.
5.1.13.3 Specific Physical Exam Findings:
Site penetration by foreign object consistent with history. In early onset, may show classic signs of
infection.
5.1.13.4 Diagnostic Testing Procedures:
X-ray, MRI, ultrasound.
5.1.13.5 Non-operative Treatment Procedures:
5.1.13.5.1 Initial
Treatment: Appropriate antibiotic therapy,
tetanus toxoid booster, non weight-bearing at physician’s discretion.
5.1.13.5.2 Medications such as analgesics and anti-inflammatories
may be helpful. Refer to medication discussions in Section 6.0, Medications and
Medical Management.
5.1.13.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.1.13.5.4 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.1.13.5.5 Other therapies in Section 6.0.
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.1.13.6 Surgical Indications/Considerations: Cellulitis, retained foreign body suspected,
abscess, compartmental syndrome, and bone involvement.
5.1.13.6.1 Smoking may affect soft tissue healing
through tissue hypoxia. Patients should be strongly encouraged to stop smoking
and be provided with appropriate counseling by the physician.
5.1.13.7 Operative Procedures:
Incision and drainage with cultures.
5.1.13.8 Post-operative Treatment:
5.1.13.8.1 Patient is usually non-weight-bearing
with antibiotic therapy based upon cultures. Follow-up x-rays and/or MRI may be
needed to evaluate for osseous involvement.
5.1.13.8.2 An individualized rehabilitation
program based upon communication between the surgeon and the therapist using
treatment as outlined in Section 6.0, Therapeutic Procedures, Non-Operative.
5.1.13.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.1.14 Severe Soft Tissue Crush Injuries:
5.1.14.1 Description/Definition: Soft tissue damage to the foot.
5.1.14.2 Occupational Relationship: Usually
from a crush injury or heavy impact to the foot or ankle.
5.1.14.3 Specific Physical Exam Findings:
Pain and swelling over the foot.
5.1.14.4 Diagnostic Testing Procedures:
X-ray and other tests as necessary to rule out other possible diagnoses
such as compartment syndrome which requires emergent compartment pressure
assessment.
5.1.14.5 Non-operative Treatment Procedures:
5.1.14.5.1 Initial Treatment: Usually needs initial rest from work with
foot elevation and compression wraps.
5.1.14.5.2 Medications such as analgesics and
anti-inflammatories may be helpful.
Refer to medication discussions in Section 6.0 Medications and Medical
Management.
5.1.14.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.1.14.5.4 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. They should
include range-of-motion (ROM), active therapies, and a home exercise program.
Active therapies include, proprioception training, restoring normal joint
mechanics, and clearing dysfunctions from adjacent structures. Passive as well as active therapies may be
used for control of pain and swelling. Therapy should progress to strengthening
and an independent home exercise program targeted to further improve ROM,
strength, and normal joint mechanics influenced by distal and proximal
structures. Refer to Section 6.0, Therapeutic Procedures, Non-operative.
5.1.14.5.4.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active
treatment. They may be used as found in
Section 6.0, Therapeutic Procedures, Non-operative.
5.1.14.5.5 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.1.14.5.6 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.1.14.6 Surgical Indications/Considerations: If compartmental pressures are elevated,
emergent fasciotomy is warranted.
5.1.14.6.1 Smoking may affect soft tissue healing
through tissue hypoxia. Patients should be strongly encouraged to stop smoking
and be provided with appropriate counseling by the physician.
5.1.14.7 Operative Procedures:
Emergency fasciotomy. In some
cases a delayed primary closure is necessary.
5.1.14.8 Post-operative Treatment:
5.1.14.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist and
using therapies as outlined in Section 6.0, Therapeutic Procedures, Non-Operative.
5.1.14.8.2 Treatment may include the following:
elevation, restricted weight-bearing, active therapy with or without passive
therapy.
5.1.14.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon or
by the surgeon.
5.1.15 Stress Fracture:
5.1.15.1 Description/Definition: Fracture without displacement usually to
metatarsals, talus, navicular or calcaneus.
5.1.15.2 Occupational Relationship:
May be related to repetitive, high impact walking; running; or jumping.
5.1.15.3 Specific Physical Exam Findings: Pain over the affected bone with
palpation or weight-bearing.
5.1.15.4 Diagnostic Testing Procedures: X-ray, CT, MRI, bone scan
5.1.15.5 Non-operative Treatment Procedures:
5.1.15.5.1 Initial Treatment: Immobilization for
4 to 8 weeks with limited weight-bearing may be appropriate.
5.1.15.5.2 Medications such as analgesics and
anti-inflammatories may be helpful.
Refer to medication discussions in Section 6.0, Medications and Medical
Management.
5.1.15.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.1.15.5.4 Refer
to comments related to osteoporosis in Section 6.0, Therapeutic
Procedures, Non-operative, Osteoporosis Management.
5.1.15.5.5 Smoking may affect fracture
healing. Patients should be strongly
encouraged to stop smoking and be provided with appropriate counseling by the
physician.
5.1.15.5.6 There is some evidence that shock
absorbing boot inserts may decrease the incidence of stress fractures in
military training. Shock absorbing boot
inserts of other orthotics may be used in some cases after a stress fracture
has occurred or to prevent stress fractures in appropriate work settings.
5.1.15.5.7 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.1.15.5.8 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.1.15.6 Surgical Indications/Considerations: Fractures that have not responded to
conservative therapy.
5.1.15.6.1 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that insurers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
5.1.15.7 Operative Procedures: Most commonly percutaneous screws or plate
fixation.
5.1.15.8 Post-operative Treatment:
5.1.15.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist and
using therapies as outlined in Section 6.0, Therapeutic Procedures, Non-Operative. In all cases, communication between the
physician and therapist is important to the timing of weight-bearing and
exercise progressions.
5.1.15.8.2 Treatment may include the following:
restricted weight-bearing, bracing, active therapy with or without passive therapy.
5.1.15.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.1.16 Talar Fracture:
5.1.16.1 Description/Definition: Osseous fragmentation of talus confirmed by
radiographic, CT or MRI evaluation.
5.1.16.2 Occupational
Relationship: Usually occurs from a
fall or crush injury.
5.1.16.3 Specific Physical Exam Findings:
Clinical findings consistent with fracture of talus: pain with range of
motion, palpation, swelling, and ecchymosis.
Pain with weight-bearing attempt.
5.1.16.4 Diagnostic Testing Procedures:
Radiographs, CT scans, MRI. CT
scans preferred for spatial alignment.
5.1.16.5 Non-operative Treatment Procedures:
5.1.16.5.1 Initial Treatment: Non weight-bearing for 6 to 8 weeks for
non-displaced fractures.
5.1.16.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
Medications and Medical Management.
5.1.16.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.1.16.5.4 Refer to comments related to
osteoporosis in Section 6.0, Therapeutic Procedures, Non-operative, Osteoporosis
Management.
5.1.16.5.5 Smoking may affect fracture healing.
Patients should be strongly encouraged to stop smoking and be provided with
appropriate counseling by the physician.
5.1.16.5.6 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.1.16.5.7 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.1.16.6 Surgical Indications/Considerations: Osseous displacement, joint involvement and
instability.
5.1.16.6.1 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that carriers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
5.1.16.7 Operative Procedures: Open
reduction internal fixation.
5.1.16.8 Post-operative Treatment:
5.1.16.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist and
using therapies as outlined in Section 6.0, Therapeutic Procedures, Non-Operative. In all cases, communication between the
physician and therapist is important to the timing of weight-bearing and
exercise progressions.
5.1.16.8.2 Treatment may include the following:
Non weight-bearing 6 to 8 weeks followed by weight-bearing cast. MRI follow-up if avascular necrosis is
suspected. Active therapy with or without passive therapy.
5.1.16.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.1.17 Tarsal Tunnel Syndrome:
5.1.17.1 Description: Pain and
paresthesias along the medial aspect of the ankle and foot due to nerve
irritation and entrapment of the tibial nerve or its branches. These symptoms can also be caused by
radiculopathy.
5.1.17.2 Occupational Relationship: Acute injuries may occur after blunt trauma
along the medial aspect of the foot.
Non-traumatic occurrences are determined at physician’s discretion after
review of environmental and biomechanical risk factors. Non work related causes include space
occupying lesions.
5.1.17.3 Specific Physical Exam Findings:
Positive Tinel's sign. Pain with
percussion of the tibial nerve radiating distally or proximally. Pain and paresthesias with weight-bearing
activities.
5.1.17.4 Diagnostic Testing Procedures:
Nerve conduction velocity studies of both sides for comparison to normal
side. EMGs may be needed to rule out
radiculopathy. MRI to rule out space
occupying lesions. Diagnostic injections to confirm the diagnosis.
5.1.17.5 Non-operative Treatment Procedures:
5.1.17.5.1 Initial Treatment: Cast or bracing, immobilization and foot
orthoses are appropriate initial management.
5.1.17.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section
6.0, Medications and Medical Management.
5.1.17.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.1.17.5.4 Return to work with appropriate
restrictions should be considered early in the course of treatment.
5.1.17.5.4.1 Orthotics or accommodative footwear
is usually necessary before workers can be returned to walking on hard
surfaces. Refer to Section 6.0, Return to Work.
5.1.17.5.5 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.1.17.6 Surgical Indications/Considerations:
5.1.17.6.1 Continued functional deficits after
active participation in therapy for 3 to 6 months.
5.1.17.6.2 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should be
especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.1.17.6.3 Smoking may affect soft tissue healing
through tissue hypoxia. Patients should be strongly encouraged to stop smoking
and be provided with appropriate counseling by the physician.
5.1.17.7 Operative Procedures: Tarsal
tunnel release with or without a plantar fascial release.
5.1.17.8 Post-operative
Treatment:
5.1.17.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist and
using therapies as outlined in Section 6.0, Therapeutic Procedures, Non-Operative.
5.1.17.8.2 Treatment may include the following:
restricted weight-bearing, orthotics, bracing, active therapy with or without
passive therapy.
5.1.17.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.1.18 Tendonopathy:
5.1.18.1 For Achilles Tendonopathy,
Refer to Section 5.1. For other types of tendonopathy of the foot and ankle,
General recommendations can be found in Section 5.2, Tendonopathy of the Knee.
5.2 KNEE
5.2.1 Aggravated
Osteoarthritis:
5.2.1.1 Description/Definition:
Swelling and/or pain in a joint due to an aggravating activity in a patient
with pre-existing degenerative change in a joint. Age greater than 50 and morning stiffness
lasting less than 30 minutes are frequently associated. The lifetime risk for symptomatic knee
arthritis is probably around 45% and is higher among obese persons.
5.2.1.2 Other causative factors to consider -
Previous meniscus or ACL damage may predispose a joint to degenerative
changes. In order to entertain previous
trauma as a cause, the patient should have medical documentation of the
following: menisectomy; hemarthrosis at the time of the original injury; or
evidence of MRI or arthroscopic meniscus or ACL damage. The prior injury should
have been at least 2 years from the presentation for the new complaints and
there should be a significant increase of pathology on the affected side in
comparison to the original imaging or operative reports and/or the opposite
un-injured side or extremity.
5.2.1.2.1 Body mass index (BMI) of 25 or
greater is a significant risk factor for eventual knee replacement.
5.2.1.3 Specific Physical Exam Findings:
Increased pain and/or swelling in a joint with joint line tenderness; joint
crepitus; and/or joint deformity.
5.2.1.4 Diagnostic
Testing Procedures:
5.2.1.4.1 Radiographs, The Kellgren-Lawrence
Scale is the standard radiographic scale for knee osteoarthritis. It is based on the development of
osteophytes, on bone sclerosis, and on joint space narrowing. The degree of joint space narrowing may not
predict disability.
5.2.1.4.1.1 Grade 1: doubtful narrowing of
joint space, and possible osteophytic lipping.
5.2.1.4.1.2 Grade 2: definite osteophytes, definite narrowing of
joint space.
5.2.1.4.1.3 Grade 3: moderate multiple osteophytes, definite
narrowing of joint space, some sclerosis and possible deformity
of bone contour.
5.2.1.4.1.4 Grade 4: large osteophytes, marked narrowing of joint
space, severe sclerosis and definite deformity of bone
contour.
5.2.1.4.2 MRI to rule out
degenerative menisci tears. MRI may identify bone marrow lesions which are
correlated with knee pain. These lesions may reflect increased water, blood, or
other fluid inside bone and may contribute to the causal pathway of pain.
These are incidental findings and should not be used to determine a
final diagnosis nor make decisions regarding surgery.
5.2.1.5 Non-operative Treatment Procedures:
5.2.1.5.1 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
Medications and Medical Management.
5.2.1.5.2 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management. There is good evidence for self-management
using weight loss, exercise, pacing of activities, unloading the joint with
braces, insoles and possibly taping, and medications as needed. Patients should be encouraged to perform
aerobic activity such as walking or biking.
However, activities such as ladders, stairs and kneeling may be
restricted.
5.2.1.5.3 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. They should
include range-of-motion (ROM), active therapies, and a home exercise program.
Active therapies include, proprioception training, restoring normal joint
mechanics, and clearing dysfunctions from distal to proximal structures.
Passive as well as active therapies may be used for control of pain and
swelling. Therapy should progress to strengthening and an independent home
exercise program targeted to further improve ROM, strength, and normal joint
mechanics influenced by structures distal and proximal to the knee. Bracing may
be appropriate in some instances. Refer to Section 6.0, Therapeutic Procedures,
Non-operative. There is good evidence
that there is a small functional advantage for patients involved in exercise
with physical therapy supervision over home exercise.
5.2.1.5.3.1 There is some evidence that active
physical therapy improves knee function more effectively than medication
alone.
5.2.1.5.3.2 Aquatic therapy may be used as a
type of active intervention when land-based therapy is not well-tolerated.
5.2.1.5.3.3 Passive modalities are most effective
as adjunctive treatments to improve the results of active treatment. They may
be used as found in Section 6.0, Therapeutic Procedures, Non-operative. There is some evidence that ice massage can
improve ROM, strengthening of the knee and function. Ice can be used with proper instruction at
home or under supervision for up to 20 minute periods 3 times per week or more
frequently.
5.2.1.5.4 Therapeutic
Injections - Both steroids and viscosupplementation may be used.
5.2.1.5.4.1 There is good evidence that
intra-articular corticosteroid injection is more effective than placebo in
reducing pain from osteoarthritis. Optimum dosage is not known.
5.2.1.5.4.2 Steroid injections may decrease
inflammation and allow the therapist to progress with functional exercise and ROM.
5.2.1.5.4.2.1 Time to Produce Effect: One
injection.
5.2.1.5.4.2.2 Maximum Duration: 3 injections
in one year at least 4 to 8 weeks apart.
5.2.1.5.4.3 Steroid injections should be used
cautiously in diabetic patients. Diabetic patients should be reminded to check
their blood glucose levels at least daily for 2 weeks after injections.
5.2.1.5.4.4 Viscosupplementation appears to
have a longer lasting effect than intra-articular corticosteroids, however, the
overall effect varies depending on the timing and the effect studied. Refer to Section 6.0, Therapeutic Procedures,
Non-operative.
5.2.1.5.5 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.2.1.5.6 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.2.1.6 Surgical Indications/Considerations:
5.2.1.6.1 Arthroscopic Debridement and/or
Lavage. There is good evidence from a randomized controlled trial that
arthroscopic debridement alone provides no benefit over recommended therapy for
patients with uncomplicated Grade 2 or higher arthritis. The comparison
recommended treatment in the study followed the American College
of Rheumatology guidelines which includes: patient education, and supervised
therapy with a home program, instruction on ADLs, stepwise use of analgesics
and hyaluronic acid injections if desired. Complicated arthritic patients
excluded from the study included patients who required other forms of
intervention due to the following associated conditions: large meniscal bucket
handle tears, inflammatory or infectious arthritis, more than 5 degrees of
varus or valgus deformity, previous major knee trauma, or Grade 4 arthritis in
2 or more compartments.
5.2.1.6.1.1 Therefore, arthroscopic
debridement and/or lavage are not recommended for patients with arthritic
findings and continual pain and functional deficits unless there is meniscal or
cruciate pathology. Refer to the specific
conditions in this Section 5.0, for specific diagnostic recommendations.
5.2.1.6.2 Osteotomy and joint
replacement are indicated when conservative treatment,
including
active participation in non-operative treatment has failed to result
insufficient functional
improvement (Refer to Section 7, Subsections regarding Knee Arthroplasty and
Osteotomy). Tibial osteotomy is a choice for younger patients with
unicompartmental disease who have failed conservative therapy.
5.2.1.6.3 In cases where surgery is
contraindicated due to obesity, it may be appropriate to recommend a weight
loss program if the patient is unsuccessful losing weight on their own. Coverage for weight loss would continue only
for motivated patients who have demonstrated continual progress with weight
loss.
5.2.1.6.4 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.2.1.6.5 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that carriers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
5.2.1.7 Operative Procedures: Total or compartmental joint replacement, and
osteotomy.
Free-floating interpositional
unicompartmental replacement is not recommended for any patients due to high
revision rate at 2 years and less than optimal pain relief.
5.2.1.8 Post-operative Treatment:
5.2.1.8.1 An individualized rehabilitation
program based upon communication between the surgeon and therapist and using
the treatments found in Section 6.0, Therapeutic Procedures,
Non-operative. In all cases,
communication between the physician and therapist is important to the timing of
weight-bearing and exercise progressions.
5.2.1.8.2 Refer also to Section 7.0, subsections
Knee Arthroplasty, or Osteotomy as appropriate.
5.2.1.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.2.2 Anterior Cruciate Ligament (ACL) Injury:
5.2.2.1 Description/Definition:
Rupture or partial rupture of the anterior cruciate ligament; may be associated
with other internal derangement of the knee.
5.2.2.2 Occupational
Relationship: May be caused by virtually any traumatic force to the knee
but most often caused by a twisting or a hyperextension force, with a valgus
stress. The foot is usually planted and
the patient frequently experiences a “popping” feeling.
5.2.2.3 Specific
Physical Exam Findings: Findings on physical exam include effusion or
hemarthrosis, instability, positive Lachman’s test, positive pivot shift test,
and positive anterior drawer test.
5.2.2.4 Diagnostic
Testing Procedures: MRI. Radiographs
may show avulsed portion of tibial spine but this is a rare finding.
5.2.2.5 Non-operative Treatment Procedures:
5.2.2.5.1 Initial
Treatment: Acute injuries may require immobilization followed by active therapy
with or without passive therapy.
5.2.2.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to Section 6.0, subsection, Medications
and Medical Management.
5.2.2.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.2.2.5.4 Benefits
may be achieved through therapeutic rehabilitation and rehabilitation
interventions. They should include range-of-motion (ROM), active therapies, and
a home exercise program. Active therapies include proprioception training,
restoring normal joint mechanics, and clearing dysfunctions from distal and
proximal structures bracing may be beneficial.
Passive as well as active therapies may be used for control of pain and
swelling. Therapy should progress to strengthening and an independent home
exercise program targeted to further improve ROM, strength, and normal joint
mechanics influenced by structures distal and proximal to the knee (Refer to
Section 6.0, Therapeutic Procedures, Non-operative). Passive modalities are
most effective as adjunctive treatments to improve the results of active
treatment. They may be used as found in Section 6.0, Therapeutic Procedures,
Non-operative.
5.2.2.5.4.1 There is no evidence that any
particular exercise regime is better for ACL injuries in combination with
collateral or meniscus injuries. There
is no evidence that knee bracing for non operated ACL improves outcomes
although patients may feel that they have greater stability. Non surgical treatment may provide acceptable
results in some patients.
5.2.2.5.5 Return to work with appropriate
restrictions should be considered
early in the course of treatment. Refer to Section 6.0, subsection Return to
Work.
5.2.2.5.6 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.2.2.6 Surgical
Indications/Considerations: any individual with complaints of
recurrent instability interfering with function
and physical findings with
imaging consistent with an ACL injury.
5.2.2.6.1 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.2.2.6.2 Smoking may affect soft tissue healing
through tissue hypoxia. Patients should be strongly encouraged to stop smoking
and be provided with appropriate counseling by the physician.
5.2.2.7 Operative Procedures:
Diagnostic/surgical arthroscopy
followed by ACL reconstruction using autograft or allograft. If meniscus repair is performed, an ACL
repair should be performed concurrently.
5.2.2.7.1 Patients tend to have more pain
associated with patellar grafts while patients with hamstring replacement seem
to have an easier rehabilitation. Choice
of graft is made by the surgeon and patient on an individual basis.
5.2.2.8 Post-operative Treatment:
5.2.2.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist and
using therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative.
5.2.2.8.2 Treatment may include the following:
active therapy with or without passive therapy and bracing. Early active extension does not cause
increased laxity at 2 years.
5.2.2.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.2.3 Bursitis
of the Lower Extremity:
5.2.3.1 Description/Definition:
Inflammation of bursa tissue. Bursitis
can be precipitated by tendonitis, bone spurs, foreign bodies, gout, arthritis,
muscle tears, or infection.
5.2.3.2 Occupational
Relationship: Usually from soft tissue trauma, contusion, or physical
activities of the job such as sustained direct compression force, or other
repetitive forceful activities affecting the knee.
5.2.3.3 Specific
Physical Exam Findings: Palpable, tender and enlarged bursa, decreased ROM,
warmth. The patient may have increased pain with ROM.
5.2.3.4 Diagnostic
Testing Procedures: Lab work may be done to rule out inflammatory
disease. Bursal fluid aspiration with
testing for connective tissue, rheumatic disease, and infection may be
necessary. Radiographs, CT, MRI are
rarely indicated.
5.2.3.5 Non-operative Treatment Procedures:
5.2.3.5.1 Initial Treatment: Diagnostic/therapeutic aspiration, ice,
therapeutic injection, treatment of an underlying infection, if present.
Aspirations may be repeated as clinically indicated.
5.2.3.5.2 medications
such as analgesics and anti-inflammatories may be helpful. Refer to medication
discussions in Section 6.0, Medications and Medical Management.
5.2.3.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.2.3.5.4 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. They should
include range-of-motion (ROM), active therapies, including a home exercise
program. Active therapies include,
proprioception training, restoring
normal joint mechanics, and clearing dysfunctions from distal and proximal
joints. Passive as well as active
therapies may be used for control of pain and swelling. Therapy should progress
to strengthening and an independent home exercise program targeted to further
improve ROM, strength, and normal joint mechanics influenced by structures
distal and proximal to the knee. Refer to Section 6.0, Therapeutic Procedures,
Non-operative.
5.2.3.5.3.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active treatment.
They may be used as found as adjunctive in Section 6.0, Therapeutic Procedures,
Non-operative.
5.2.3.5.5 Steroid Injections- Steroid
injections may decrease inflammation and allow the therapist to progress with
functional exercise and ROM.
5.2.3.5.5.1 Time to Produce Effect: One
injection.
5.2.3.5.5.2 Maximum Duration: 3 injections in
one year spaced at least 4 to 8 weeks apart.
5.2.3.5.5.3 Steroid injections should be used
cautiously in diabetic patients. Diabetic patients should be reminded to check
their blood glucose levels at least daily for 2 weeks after injections.
5.2.3.5.6 Return to work with appropriate
restrictions should be considered
early in the course of treatment. Refer to Section 6.0. subsection Return to
Work.
5.2.3.5.7 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.2.3.6 Surgical indications/Considerations:
5.2.3.6.1 Failure of conservative
therapy.
5.2.3.6.2 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.2.3.6.3 Smoking may affect soft tissue
healing through tissue hypoxia. Patients should be strongly encouraged to stop
smoking and be provided with appropriate counseling by the physician.
5.2.3.7 Operative Procedures:
Surgical excision of the bursa.
5.2.3.8 Post-operative Treatment:
5.2.3.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist and
using the therapies as outlined in Section 6.0, Therapeutic Procedures, Non-operative.
5.2.3.8.2 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.2.4 Chondral
and Osteochondral Defects:
5.2.4.1 Description/Definition: Cartilage or cartilage and bone defect at the
articular surface of a joint. Deficits
may be identified in up to 60% of arthroscopies; however, only around 30% of
these lesions are isolated deficits and even fewer are Grade III or IV deficits
which might qualify for cartilage grafts.
5.2.4.1.1 Defects in cartilage and bone are
common at the femoral condyles and patella. The Outerbridge classification
grades these defects according to their size and depth.
5.2.4.1.1.1 Grade 0: normal
cartilage.
5.2.4.1.1.2 Grade I: softening and swelling of cartilage.
5.2.4.1.1.3 Grade
II: partial-thickness defects with
surface fissures that do not exceed .5 cm in diameter and do not reach
subchondral bone.
5.2.4.1.1.4 Grade
III: fissuring that reaches subchondral bone in an area with a diameter greater
than 1.5 cm.
5.2.4.1.1.5 Grade IV: exposed
subchondral bone.
5.2.4.2 Occupational
Relationship: Typically caused by a traumatic knee injury. Chondral deficits can also be present
secondary to osteoarthritis.
5.2.4.3 Specific Physical Exam Findings:
Knee effusion, joint line tenderness.
5.2.4.4 Diagnostic
Testing Procedures: MRI may show bone bruising, osteochondral lesion, or
possibly articular cartilage injury.
Radiographs, contrast radiography, CT may also be used. Diagnostic arthroscopy may be performed when
surgical indications as stated in Section VI are met.
5.2.4.5 Non-operative Treatment Procedures:
5.2.4.5.1 Initial Treatment: Non-operative treatment may be indicated for
chondral lesions associated with 1) degenerative changes, refer to aggravated
osteoarthritis (Section 5.0); 2) other knee lesions not requiring surgery
(refer to Specific Diagnosis); and/or 3) non-displaced stable lesions. Acute injuries may require immobilization
followed by active therapy with or without passive therapy.
5.2.4.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
Medications and Medical Management.
5.2.4.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.2.4.5.4 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. They should
include range-of-motion (ROM), active therapies, and a home exercise program.
Active therapies include, proprioception training, restoring normal joint
mechanics, and clearing dysfunctions from distal and proximal structures. Passive as well as active therapies may be
used for control of pain and swelling. Therapy should progress to strengthening
and an independent home exercise program targeted to further improve ROM,
strength, and normal joint mechanics influenced by structures distal and
proximal to the knee. Refer to Section 6.0, Therapeutic Procedures,
Non-operative.
5.2.4.5.4.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active
treatment. They may be used as found in
Section 6.0, Therapeutic Procedures, Non-operative.
5.2.4.5.5 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.2.4.5.6 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.2.4.6 Surgical
Indications/Considerations: Surgery
for isolated chondral defects may be indicated when functional deficits
interfere with activities of daily living and/or job duties after 6 to 12 weeks
of active patient participation in non-operative therapy. Identification of the
lesion should have been accomplished by diagnostic testing procedures which
describe the size of the lesion and stability of the joint. If a lesion is detached or has fluid underlying
the bone on MRI, surgery may be necessary before a trial of conservative
therapy is completed. Early surgery may consist of fixation or microfracture.
5.2.4.6.1 Microfractures: Normally
the first line of surgical treatment.
5.2.4.6.1.1 Indications: An isolated small full-thickness articular
chondral defect with normal joint space, when the patient has not recovered
functionally after active participation in therapy. Patients 45 or younger are likely to have
better results.
5.2.4.6.2 Osteochondral Autograft
Transfer System (OATS)
5.2.4.6.2.1 Indications:
The knee must be stable with intact ligaments and menisci, normal joint space
and a large full-thickness defect less than 3 square cm and 1 cm depth. They should be 45 or younger, with a BMI less
than 35, and engaged in athletics and/or an equally physically demanding
occupation. Surgery may be indicated
when functional deficits interfere with activities of daily living and/or job
duties after 6 to 12 weeks of active patient participation in non-operative
therapy. This procedure may be
appropriate in a small subset of patients and requires prior authorization.
5.2.4.6.3 Autologous chondrocyte implantation
(ACI): These procedures are technically difficult and require specific
physician expertise. Cartilage
transplantation requires the harvesting and growth of patients’ cartilage cells
in a highly specialized lab and incurs significant laboratory charges. There is
some evidence that transplants and microfractures do not differ on long-term
effects. There is some evidence that
autologous chrondrocyte implantation is not better than microfracture 5 years
after surgery in patients younger than 45 presenting with Grade III -IV
lesions. This procedure is controversial
but may be appropriate in a small subset of patients with physically rigorous
employment or recreational activities.
It requires prior authorization.
5.2.4.6.3.1 Indications: The area of the lesion should be between 2
square cm and 10 square cm. The patient should have failed 4 or more months of
active participation in therapy and a
microfracture, abrasion, arthroplasty or drilling with sufficient healing time,
which may be from 4 months to over one year.
The knee must be stable with intact ligaments and meniscus, and normal
joint space. Patients should be 45 or
younger, with a BMI less than 35, and engaged in athletics and/or an equally
physically demanding occupation.
5.2.4.6.4 Contraindications: General contraindications for grafts and
transplants are individuals with obesity, inflammatory or osteoarthritis with multiple
chondral defects, associated ligamentous or meniscus pathology, or who are
older than 55 years of age.
5.2.4.6.5 Prior to either graft or implantation
intervention the patient and treating physician should identify functional
operative goals and the likelihood of achieving improved ability to perform
activities of daily living or work activities and the patient should agree to
comply with the pre- and post-operative treatment plan including home exercise.
The provider should be especially careful to make sure the patient understands
the amount of post-operative therapy required and the length of partial- and
full-disability expected post-operatively.
5.2.4.6.6 Smoking may affect soft tissue
healing through tissue hypoxia. Patients should be strongly encouraged to stop
smoking and be provided with appropriate counseling by the physician.
5.2.4.7 Operative
Procedures: Arthroscopy with debridement or shaving of cartilage,
microfracture, drilling, abrasion arthroplasty, mosiacplasty or osteochondral
autograft (OATS), fixation of loose osteochondral fragments and autologous
chondrocyte implantation (ACI).
5.2.4.7.1 Radiofrequency
treatment is not recommended.
5.2.4.8 Post-operative Treatment:
5.2.4.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist and
using therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative. In all cases,
communication between the physician and therapist is important to the timing of
weight-bearing and exercise progressions.
5.2.4.8.2 Treatment may include the following:
restricted weight-bearing, bracing, active therapy with or without passive
therapy. Full weight-bearing usually
occurs by or before 8 weeks.
5.2.4.8.3 Continuous passive motion
may be used after chondral procedures.
5.2.4.8.4 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon. Return to full-duty usually occurs by between four and six
months.
5.2.5 Collateral
Ligament Pathology:
5.2.5.1 Description/Definition: Strain or tear
of medial or lateral collateral ligaments
which provide some stabilization for the knee.
5.2.5.2 Occupational
Relationship: Typically a result of forced abduction and external rotation
to an extended or slightly flexed knee.
5.2.5.3 Specific
Physical Exam Findings: Swelling or ecchymosis over the collateral
ligaments and increased laxity or pain with applied stress.
5.2.5.4 Diagnostic
Testing Procedures: X-rays to rule out fracture. Imaging is more commonly ordered when
internal derangement is suspected.
5.2.5.5 Non-operative Treatment Procedures:
5.2.5.5.1 Initial Treatment: braces, ice, and protected weight-bearing.
5.2.5.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions area in
Section 6.0, Medications and Medical Management.
5.2.5.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.2.5.5.4 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. They should
include range-of-motion (ROM), active therapies, and a home exercise program.
Active therapies include proprioception training, restoring normal joint
mechanics, and clearing dysfunctions from distal and proximal structures.
Bracing may be beneficial. Passive as
well as active therapies may be used for control of pain and swelling. Therapy
should progress to strengthening and an independent home exercise program
targeted to further improve ROM, strength, and normal joint mechanics
influenced by structures distal and proximal to the knee. Refer to Section 6.0
Therapeutic Procedures, Non-operative.
5.2.5.5.4.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active
treatment. They may be used as found in
Section 6.0 Therapeutic Procedures, Non-operative.
5.2.5.5.5 Return
to work with appropriate restrictions should be considered early in the course
of treatment. Refer to Section 6.0, Return to Work.
5.2.5.5.6 Other
therapies in Section 6.0, Therapeutic Procedures, Non-operative may be employed
in individual cases.
5.2.5.6 Surgical
Indications/Considerations: Surgery
is rarely necessary except when functional instability persists after active
participation in non-operative treatment or indications for surgery exist due
to other accompanying injuries.
5.2.5.6.1 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.2.5.6.2 Smoking may affect soft tissue
healing through tissue hypoxia. Patients should be strongly encouraged to stop
smoking and be provided with appropriate counseling by the physician.
5.2.5.7 Operative Procedures: Surgical repair.
5.2.5.8 Post-operative Treatment:
5.2.5.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist and
using procedures as outlined in Section 6.0, Therapeutic Procedures,
Non-operative.
5.2.5.8.2 Return to
work and restrictions after surgery may be made by an attending physician in
consultation with the surgeon or by the surgeon.
5.2.6 Meniscus Injury:
5.2.6.1 Description/Definition:
A tear, disruption, or avulsion of medial or lateral meniscus tissue. Locking of the knee or clicking is frequently
reported. Patients may describe a
popping, tearing, or catching sensation followed by stiffness.
5.2.6.2 Occupational
Relationship: Usually, trauma to the menisci stems from rotational
shearing, torsion, and/or impact injuries while in a flexed position.
5.2.6.3 Specific
Physical Exam Findings: Joint line tenderness, Positive McMurray’s test
locked joint, or occasionally, effusion.
The presence of joint line tenderness has a sensitivity of 85% and a
specificity of 31%. The Apley’s
compression test is also used.
5.2.6.4 Diagnostic
Testing Procedures: Radiographs including standing Posterior/Anterior (PA),
lateral, tunnel, and skyline views. MRI
is the definitive imaging test. MRI is
sensitive and specific for meniscal tear.
However, meniscal MRI is frequently abnormal in asymptomatic
injuries. In one study of volunteers
without a history of knee pain, swelling, locking, giving way, or any knee
injury, 16% of the volunteers had MRI-evident meniscal tears; among volunteers
older than 45, 36% had MRI-evident meniscal tears. Therefore, clinical correlation with history
and physical exam findings specific for meniscus injury is critically
important.
5.2.6.4.1 Providers planning treatment should
therefore consider the patient's complaints and presence of arthritis on MRI
carefully, knowing that not all meniscus tears in the middle aged and older
population are related to the patients’ complaints of pain.
5.2.6.4.2 MRI arthrograms are used to diagnose
recurrent meniscal tears particularly after previous surgery.
5.2.6.5 Non-operative Treatment:
5.2.6.5.1 Initial Treatment: ice,
bracing, and protected weight-bearing.
5.2.6.5.2 Medications
such as analgesics and anti-inflammatories may be helpful. Refer to medication
discussions in Section 6.0, Medications and Medical Management.
5.2.6.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.2.6.5.4 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation Interventions. They should
include range-of-motion (ROM), active therapies, and a home exercise
program. Active therapies include,
proprioception training, restoring normal
joint mechanics, and clearing dysfunctions from distal and proximal structures.
Passive as well as active therapies may be used for control of pain and
swelling. Therapy should progress to strengthening and an independent home
exercise program targeted to further improve ROM, strength, and normal joint
mechanics influenced by structures distal and proximal to the knee. Refer to
Section 6.0 Therapeutic Procedures, Non-operative.
5.2.6.5.4.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active treatment.
They may be used as found as adjunctive in Section 6.0, Therapeutic Procedures,
Non-operative.
5.2.6.5.5 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work Subsection.
5.2.6.5.6 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.2.6.6 Surgical
Indications/Considerations: 1.
Locked or blocked knee precluding active therapy; 2. Isolated acute meniscus
tear with appropriate physical exam findings; 3. Meniscus pathology combined
with osteoarthritis in a patient with functional deficits interfering with
activities of daily living and/or job duties after 6 to 12 weeks of active
patient participation in non-operative therapy.
5.2.6.6.1 It is not clear that partial
meniscectomy for a chronic degenerative meniscal tear is beneficial. Middle aged patients may do as well without
arthroscopy and with therapy.
5.2.6.6.2 Meniscal allograft should only be
performed on patients between 20 and 45 with an otherwise stable knee, previous
meniscectomy with 2/3 removed, lack of function despite active therapy, BMI
less than 35, and sufficient joint surface to support repair.
5.2.6.6.3 Medial collagen meniscus implants are
considered experimental and not generally recommended. No studies have been done to compare this
procedure to medial meniscus repair. There is some evidence to support the fact
that collagen meniscal implant may slightly improve function and decrease risk
of reoperation in patients with previous medial meniscal surgery. It remains unclear as to the extent that the
procedure may decrease future degenerative disease. The procedure can only be considered for
individuals with previous medial meniscal surgery and intact meniscus rim;
without lateral meniscus lesions or Grade 4 Outerbridge lesions; and who need
to return to heavy physical labor employment or demanding recreational
activities. A second concurring opinion from an orthopedic surgeon specializing
in knee surgery and prior authorization is required. Full weight-bearing is not allowed for 6
weeks and most patients return to normal daily activity after 3 months.
5.2.6.6.4 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.2.6.6.5 Smoking may affect soft tissue
healing through tissue hypoxia. Patients should be strongly encouraged to stop
smoking and be provided with appropriate counseling by the physician.
5.2.6.7 Operative
Treatment: Repair of meniscus, partial or complete excision of meniscus or
meniscus allograft or implant. Debridement of the meniscus is not recommended
in patients with severe arthritis as it is unlikely to alleviate symptoms. Complete excision of meniscus should only be
performed when clearly indicated due to the long-term risk of arthritis in
these patients. Partial meniscectomy or meniscus repair is preferred to total
meniscectomy due to easier recovery, less instability, and short-term
functional gains.
5.2.6.8 Post-operative Treatment:
5.2.6.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist using
the treatments found in Section 6.0, Therapeutic Procedures, Non-operative.
5.2.6.8.2 Treatment may include the following:
Passive therapy progressively moving toward active therapy, bracing, cryotherapy and other
treatments found in Section 6.0.
5.2.6.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.2.7 Patellar Fracture:
5.2.7.1 Description/Definition:
Fracture of the patella.
5.2.7.2 Occupational Relationship: Usually from a traumatic injury such as
a fall or direct blow
5.2.7.3 Specific
Physical Exam Findings: Significant hemarthrosis/effusion usually present.
Extension may be limited and may indicate disruption of the extensor
mechanism. It is essential to rule out
open fractures; therefore a thorough search for lacerations is important.
5.2.7.4 Diagnostic
Testing Procedures: Aspiration of
the joint and injection of local anesthetic may aid the diagnosis. A saline
load injected in the joint can also help rule out an open joint injury. Radiographs may be performed, including
tangential (sunrise) or axial views and x-ray of the opposite knee in many
cases. CT or MRI is rarely needed.
5.2.7.5 Non-operative Treatment Procedures:
5.2.7.5.1 Initial Treatment: For non-displaced
closed fractures, protected weight-bearing and splinting for 4 to 6 weeks.
Hinged knee braces can be used. When radiographs demonstrate consolidation,
active motion and strengthening exercise may begin.
5.2.7.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section
6.0, Medications and Medical Management.
5.2.7.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.2.7.5.4 Refer to comments related to
osteoporosis in Section 6.0, Therapeutic Procedures, Non-operative,
Osteoporosis Management.
5.2.7.5.5 Smoking may affect fracture healing.
Patients should be strongly encouraged to stop smoking and be provided with
appropriate counseling by the physician.
5.2.7.5.6 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions, after boney union
has been achieved. They should include bracing then range-of-motion (ROM),
active therapies including proprioception training, restoring normal joint
mechanics, and clearing dysfunctions from adjacent structures, and a home
exercise program. Passive as well as active therapies may be used for control
of pain and swelling. Therapy should progress to strengthening and an
independent home exercise program targeted to further improve ROM, strength,
restoring normal joint mechanics, influenced by proximal and distal structures.
Therapy should include training on the use of adaptive equipment and home and
work site evaluations when appropriate.
Bracing may be appropriate. Refer to Section 6.0, Therapeutic
Procedures, Non-operative.
5.2.7.5.6.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active treatment.
They may be used as found as adjunctive in Section 6.0, Therapeutic Procedures,
Non-operative.
5.2.7.5.7 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.2.7.5.8 Other therapies in Section 6.0,
Therapeutic Procedures, Non-Operative may be employed in individual cases.
5.2.7.6 Surgical
Indications/Considerations: Open fractures require immediate intervention
and may need repeat debridement. Internal fixation is usually required for
comminuted or displaced fractures.
Non-union may also require surgery.
5.2.7.6.1 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that insurers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
5.2.7.7 Operative
Procedures: internal fixation; partial patellectomy or total patellectomy.
Total patellectomy results in instability with running or stairs and
significant loss of extensor strength.
Therefore, this is usually a salvage procedure.
5.2.7.8 Post-operative Treatment:
5.2.7.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist and
using therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative. In all cases,
communication between the physician and therapist is important to the timing of
weight-bearing and exercise progressions.
Continuous passive motion may be used post operatively.
5.2.7.8.2 Treatment may include protected
weight-bearing and active therapy with or without passive therapy for early range
of motion if joint involvement.
5.2.7.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.2.7.8.4 Hardware removal may be
necessary after 3 to 6 months.
5.2.8 Patellar
Subluxation:
5.2.8.1 Description/Definition: Incomplete subluxation or dislocation of the
patella. Recurrent episodes can lead to
subluxation syndrome that can cause frank dislocation of the patella. Patient
may report a buckling sensation, pain with extension, or a locking of the knee
with exertion.
5.2.8.2 Occupational
Relationship: Primarily associated
with a direct contact lateral force. Secondary causes associated with shearing
forces on the patella.
5.2.8.3 Specific
Physical Exam Findings: Lateral retinacular tightness with associated
medial retinacular weakness, swelling, effusion, and marked pain with
patellofemoral tracking/compression and glides.
In addition, other findings may include atrophy of muscles, positive
patellar apprehension test, and patella alta.
5.2.8.4 Diagnostic
Testing Procedures: CT or
Radiographs including Merchant views, Q-angle, and MRI for loose bodies.
5.2.8.5 Non-operative Treatment Procedures:
5.2.8.5.1 Initial Treatment: Reduction if necessary, ice, taping, and
bracing followed by active therapy.
5.2.8.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
Medications and Medical Management.
5.2.8.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.2.8.5.4 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. They should
include range-of-motion (ROM), active therapies, and a home exercise program.
Active therapies include, proprioception training, restoring normal joint
mechanics, and clearing dysfunctions from distal and proximal structures. Taping the patella or bracing may be beneficial.
Passive as well as active therapies can be used for control of pain and
swelling. Therapy should progress to
strengthening and an independent home exercise program targeted to further
improve ROM, strength, and normal joint mechanics influenced by structures
distal and proximal to the knee.
Specific strengthening should be done to optimize patellofemoral
mechanics and address distal foot mechanics that influence the patellofemoral
joint. Refer to Section 6.0, Therapeutic Procedures, Non-operative.
5.2.8.5.4.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active treatment.
They may be used as found in Section 6.0, Therapeutic Procedures,
Non-operative.
5.2.8.5.5 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.2.8.5.6 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.2.8.6 Surgical Indications/Considerations:
5.2.8.6.1 Fracture, loose bodies, and recurrent
dislocation. Surgical repair of first-time dislocation in young adults
generally is not recommended. Retinacular release, quadriceps reefing, and
patellar tendon transfer should only be considered for subluxation after 4 to 6
months of active patient participation in non-operative treatment.
5.2.8.6.2 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.2.8.6.3 Smoking may affect soft tissue
healing through tissue hypoxia. Patients should be strongly encouraged to stop
smoking and be provided with appropriate counseling by the physician.
5.2.8.7 Operative
Procedures: arthroscopy with possible arthrotomy; debridement of soft
tissue and articular cartilage disruption; open reduction internal fixation
with fracture; retinacular release, quadriceps reefing, and patellar tendon or
lateral release with or without medial soft-tissue realignment.
5.2.8.8 Post-operative Treatment:
5.2.8.8.1 Individualized rehabilitation program
based upon communication between the surgeon and the therapist using the
treatments found in Section 6.0, Therapeutic Procedures, Non-operative.
5.2.8.8.2 Treatment may include
active therapy with or without passive therapy, bracing.
5.2.8.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.2.9 Patellofemoral Pain Syndrome (aka Retropatellar Pain Syndrome):
5.2.9.1 Description/Definition:
Patellofemoral pathologies are associated with resultant weakening,
instability, and pain of the patellofemoral mechanism. Diagnoses can include
patellofemoral chondromalacia, malalignment, persistent quadriceps tendonitis,
distal patellar tendonitis, patellofemoral arthrosis, and symptomatic plica
syndrome. Patient complains of pain, instability and tenderness that interfere
with daily living and work functions such as sitting with bent knees, climbing
stairs, squatting, running or cycling.
5.2.9.2 Occupational
Relationship: Usually associated
with contusion; repetitive patellar compressive forces; shearing articular
injuries associated with subluxation or dislocation of patella, fractures,
and/or infection.
5.2.9.3 Specific
Physical Exam Findings: Findings on physical exam may include retinacular
tenderness, pain with patellar compressive ranging, positive patellar glide
test, atrophy of quadriceps muscles, positive patellar apprehensive test. Associated anatomical findings may include
increased Q angle; ligament laxity, and effusion. Some studies suggest that the patellar tilt
test (assessing the patella for medial tilt) and looking for active instability
with the patient supine and knee flexed to 15 degrees and an isometric quad
contraction, may be most useful for distinguishing normal from abnormal. Most patellar tests are more specific than
sensitive.
5.2.9.4 Diagnostic
Testing Procedures: Radiographs including tunnel view, axial view of
patella at 30 degrees, lateral view and Merchant views. MRI rarely identifies pathology. Occasional
CT or bone scans.
5.2.9.5 Non-operative Treatment Procedures:
5.2.9.5.1 Medications
such as analgesics and anti-inflammatories may be helpful. Refer to medication
discussions in Section 6.0, Medications and Medical Management.
5.2.9.5.2 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.2.9.5.3 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. The program should
include bracing and/or patellar taping, prone quad stretches, hip external
rotation, balanced strengthening, range-of-motion (ROM), active therapies and a
home exercise program. Active therapies
include proprioception training,
restoring normal joint mechanics, and clearing dysfunctions from distal and
proximal structures. Passive as well as
active therapies may be used for control of pain and swelling. Active
therapeutic exercise appears to decrease pain; however, the expected functional
benefits are unclear. Therapy should progress
to strengthening and an independent home exercise program targeted to further
improve ROM strength, and normal joint mechanics influenced by structures
distal and proximal to the knee. Refer
to Section 6.0, Therapeutic Procedures, Non-operative.
5.2.9.5.3.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active treatment.
They may be used as found as adjunctive in Section 6.0, Therapeutic Procedures,
Non-operative. Orthotics may be useful
in some cases.
5.2.9.5.4 Knee pain, when associated with
abnormal foot mechanics, may be favorably treated with appropriate orthotics.
5.2.9.5.4.1 There is some evidence that
pre-fabricated commercially available foot orthotic devices are more beneficial
for patients with patellofemoral pain syndrome than flat shoe inserts. They may
produce mild side effects such as rubbing or blistering which can be reduced
with additional empirical measures such as heat molding or addition, and
removal of wedges and inserts until patient comfort is achieved. In some cases,
custom semi-rigid or rigid orthotics is necessary to decrease pronation or
ensure a proper fit.
5.2.9.5.5 Botulinum toxin injections for the
relief of patellofemoral pain are considered experimental and are not recommended.
5.2.9.5.6 Steroid Injections:
5.2.9.5.6.1 Steroid injections may decrease
inflammation and allow the therapist to progress with functional exercise and ROM. Steroid injections under significant pressure
should be avoided as the needle may be penetrating the tendon and injection
into the tendon can cause possible tendon breakdown, tendon degeneration, or
rupture. Injections near the patellar
tendon should generally be avoided.
Injections should be minimized for patients less than 30 years of age.
5.2.9.5.6.2 Time to Produce Effect: One
injection.
5.2.9.5.6.3 Maximum Duration: 3 injections in
one year spaced at least 4 to 8 weeks apart.
5.2.9.5.6.4 Steroid injections should be used
cautiously in diabetic patients. Diabetic patients should be reminded to check
their blood glucose levels at least daily for 2 weeks after injections. The injection may be performed with or
without ultrasound guidance. Ultrasound
guided interventional procedures provides the ability to image soft tissues in
real time and can improve safety and accuracy of needle placement. The use of ultrasound guided procedures will
be at the discretion of the health care provider.
5.2.9.5.7 Extracorporeal Shock Wave Therapy
(ESWT): There is no good research to support ESWT and therefore, it is
not recommended.
5.2.9.5.8 Return
to work with appropriate restrictions should be considered early in the course
of treatment. Refer to Section 6.0, Return to Work.
5.2.9.5.9 Other therapies in Section 6.0,
Therapeutic Procedures, Non-Operative may be employed in individual cases.
5.2.9.6 Surgical
Indications/Considerations: patellar tendon disruption, quadriceps tendon
rupture/avulsion, fracture. There is no evidence that surgery is better than
eccentric training for patellar tendonopathy of the inferior pole (jumper’s
knee).
5.2.9.6.1 Retinacular release, quadriceps
reefing, and tibial transfer procedures should only be considered after 4 to 6
months of active patient participation in non-operative treatment in young
active patients. There is no evidence
that arthroscopy for patellofemoral syndrome is more efficacious than
exercise.
5.2.9.6.2 Lateral release and reconstruction is
not recommended for patellofemoral arthritis or middle aged adults.
5.2.9.6.3 In cases of severe Grade III-IV
isolated patellofemoral arthritis where walking, steps, and other functional
activities are significantly impacted after adequate conservative treatment,
prosthesis may be considered in those less than 55 years. A patellofemoral
arthroplasty is generally contraindicated if there is patellofemoral
instability or malalignment, tibiofemoral mechanical malalignment, fixed loss
of knee motion (greater than 10 degrees extension or less than 110 degrees
flexion), inflammatory arthritis, and other systemic related issues. For patellar resurfacing, refer to Section 7.0,
Knee Arthroplasty.
5.2.9.6.4 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.2.9.6.5 Smoking may affect soft tissue
healing through tissue hypoxia. Patients should be strongly encouraged to stop
smoking and be provided with appropriate counseling by the physician.
5.2.9.7 Operative
Procedures: Arthroscopic debridement of articular surface, plica, synovial
tissue, loose bodies; arthrotomy; open reduction internal fixation with
fracture; patellar prosthesis with isolated Grade III-IV OA, and possible
patellectomy for young active patients with isolated arthritis.
5.2.9.8 Post-operative Treatment:
5.2.9.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist and
using therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative.
5.2.9.8.2 Treatment may include active therapy
with or without passive therapy; and bracing.
5.2.9.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.2.10 Posterior Cruciate Ligament (PCL) Injury:
5.2.10.1 Description/Definition: Rupture of PCL. May be associated with
concurrent ACL rupture or collateral ligament injury.
5.2.10.2 Occupational Relationship:
Most often caused by a posterior force directed to flexed knee.
5.2.10.3 Specific Physical Exam Findings:
Findings on physical exam include acute effusion, instability, reverse
Lachman’s test, reverse pivot shift, posterior drawer test.
5.2.10.4 Diagnostic Testing Procedures:
MRI, radiographs including kneeling view, may reveal avulsed bone.
5.2.10.5 Non-operative Treatment Procedures:
5.2.10.5.1 Initial Treatment: Ice, bracing, and protected weight-bearing
followed by active therapy.
5.2.10.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
Medications and Medical Management.
5.2.10.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.2.10.5.4 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. They should
include bracing then range-of-motion (ROM), active therapies, and a home
exercise program. Active therapies include proprioception training, restoring
normal joint mechanics, and clearing dysfunctions from distal and proximal
structures. Passive as well as active therapies may be used for control of pain
and swelling. Therapy should progress to strengthening and an independent home
exercise program targeted to further improve ROM, strength, and normal joint
structures distal and proximal to the knee. Refer to Section 6.0, Therapeutic
Procedures, Non-operative.
5.2.10.5.4.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active treatment.
They may be used as found in Section 6.0, Therapeutic Procedures, Non-operative.
5.2.10.5.5 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.2.10.5.6 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.2.10.6 Surgical Indications/Considerations:
5.2.10.6.1 Carefully consider the patients’
normal daily activity level before initiation of surgical intervention.
Isolated Grade 1 instability does not require surgical intervention. Grades 2
or 3 may have surgical intervention if there remains demonstrable instability
which interferes with athletic or work pursuits of the patient. In a second degree sprain there is
significant posterior motion of the tibia on the femur in active testing. A third degree sprain demonstrates rotary
instability due to medial or lateral structural damage. Surgery is most commonly done when the PCL rupture is accompanied by multi-ligament
injury. Not recommended as an isolated procedure in patients over 50 with Grade
3 or 4 osteoarthritis.
5.2.10.6.2 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.2.10.6.3 Smoking may affect soft tissue healing
through tissue hypoxia. Patients should be strongly encouraged to stop smoking
and be provided with appropriate counseling by the physician.
5.2.10.7 Operative Procedures: Autograft or allograft reconstruction.
5.2.10.8 Post-operative Treatment:
5.2.10.8.1 An individualized rehabilitation
program based upon communication between the surgeon/physician and the therapy
provider and using therapies as outlined in Section 6.0, Therapeutic
Procedures, Non-operative.
5.2.10.8.2 Treatment
may include active therapy with or without passive therapy, bracing.
5.2.10.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.2.11 Tendonopathy:
5.2.11.1 Description/Definition: Inflammation of the lining of the tendon
sheath or of the enclosed tendon.
Usually occurs at the point of insertion into bone or a point of
muscular origin. Can be associated with bursitis, calcium deposits, or systemic
connective diseases.
5.2.11.2 Occupational Relationship: Usually from extreme or repetitive
trauma, strain, or excessive unaccustomed exercise or work.
5.2.11.3 Specific Physical Exam Findings: Involved tendons may be visibly
swollen with possible fluid accumulation and inflammation; popping or crepitus;
and decreased ROM.
5.2.11.4 Diagnostic Testing Procedures: Lab work may be done to rule out
inflammatory disease. Other tests are
rarely indicated.
5.2.11.5 Non-operative Treatment Procedures:
5.2.11.5.1 Initial Treatment: Ice, protected weight-bearing and/or
restricted activity, possible taping and/or bracing.
5.2.11.5.2 Medications
such as analgesics and anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
Medications and Medical Management.
5.2.11.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.2.11.5.4 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. They should
include range-of-motion (ROM), active therapies, including a home exercise
program. Active therapies include, proprioception
training, restoring normal joint mechanics, and clearing dysfunctions from
distal and proximal structures. Passive
as well as active therapies may be used for control of pain and swelling.
Therapy should progress to strengthening and an independent home exercise
program targeted to further improve ROM, strength, and normal joint mechanics
influenced by structures distal and proximal to the knee. Refer to Section 6.0,
Therapeutic Procedures, Non-operative.
5.2.11.5.4.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active treatment.
They may be used as found as adjunctive in Section 6.0, Therapeutic Procedures,
Non-operative.
5.2.11.5.5 For isolated patellar tendonopathy,
patellar tendon strapping or taping may be appropriate.
5.2.11.5.6 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.2.11.5.7 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.2.11.5.8 Therapeutic Injections:
5.2.11.5.8.1 Steroid
injections may decrease inflammation and allow the therapist to progress with
functional exercise and ROM. Steroid injections under significant pressure
should be avoided as the needle may be penetrating the tendon and injection
into the tendon can cause possible tendon breakdown, tendon degeneration, or
rupture. Injections should be minimized
for patients less than 30 years of age.
5.2.11.5.8.1.1 Time to Produce Effect: One
injection.
5.2.11.5.8.1.2 Maximum Duration: 3 injections
in one year spaced at least 4 to 8 weeks apart.
5.2.11.5.8.1.3 Steroid injections should be
used cautiously in diabetic patients. Diabetic patients should be reminded to
check their blood glucose levels at least daily for 2 weeks after injections. All injections may be performed with or
without ultrasound guidance. Ultrasound
guided interventional procedures provides the ability to image soft tissues in
real time and can improve safety and accuracy of needle placement. The use of ultrasound guided procedures will
be at the discretion of the health care provider.
5.2.11.6 Surgical Indications/Considerations:
5.2.11.6.1 Suspected avulsion fracture, or severe
functional impairment unresponsive to a minimum of 4 months of active patient
participation in non-operative treatment.
5.2.11.6.2 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.2.11.6.3 Smoking may affect soft tissue healing
through tissue hypoxia. Patients should be strongly encouraged to stop smoking
and be provided with appropriate counseling by the physician.
5.2.11.7 Operative Procedures: Tendon
repair. Rarely indicated and only after
extensive conservative therapy.
5.2.11.8 Post-operative Treatment:
5.2.11.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist and
using therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative.
5.2.11.8.2 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.3 HIP AND LEG
5.3.1 Acetabular Fracture:
5.3.1.1 Description/Definition:
Subgroup of pelvic fractures with involvement of the hip articulation.
5.3.1.2 Occupational Relationship: Usually from a traumatic injury such as
a fall or crush.
5.3.1.3 Specific
Physical Exam Findings: Displaced fractures may have short and/or
abnormally rotated lower extremity.
5.3.1.4 Diagnostic Testing Procedures: Radiographs, CT scanning.
5.3.1.5 Non-operative Treatment Procedures:
5.3.1.5.1 Initial Treatment: Although surgery
is frequently required, protected weight-bearing may be considered for
un-displaced fractures or minimally displaced fractures that do not involve the
weight-bearing surface of the acetabular dome.
5.3.1.5.2 Medications such as analgesics and
anti-inflammatories may be helpful.
Refer to medication discussions in Section 6.0, Medications and Medical
Management.
5.3.1.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home exercise,
joint protection, and weight management.
5.3.1.5.4 Refer to comments on
osteoporosis in Section 5.0, Ankle Sprain/Fracture.
5.3.1.5.5 Smoking may affect fracture healing.
Patients should be strongly encouraged to stop smoking and be provided with
appropriate counseling by the physician.
5.3.1.5.6 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions, after boney union
has been achieved. They should include bracing then range-of-motion (ROM),
active therapies, and a home exercise program.
Active therapies include ambulation with appropriate assistive device,
proprioception training, restoring normal joint mechanics, and clearing
dysfunctions from adjacent structures. Passive as well as active therapies may
be used for control of pain and swelling. Therapy should progress to
strengthening and an independent home exercise program targeted to further
improve ROM, strength, and normal joint mechanics influenced by proximal and
distal structures. Therapy should include training on the use of adaptive
equipment and home and work site evaluations when appropriate. Bracing may be appropriate. Refer to Section 6.0,
Therapeutic Procedures, Non-operative.
5.3.1.5.6.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active treatment.
They may be used as found as adjunctive in Section 6.0, Therapeutic Procedures,
Non-operative.
5.3.1.5.7 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.3.1.5.8 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.3.1.6 Surgical Indications/Considerations: Displaced or unstable
fracture.
5.3.1.6.1 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that insurers cover a
smoking cessation program peri-operatively. Physicians may monitor smoking
cessation with laboratory tests such as cotinine levels for long-term
cessation.
5.3.1.7 Operative
Procedures: Usually open reduction and internal fixation or total hip
replacement.
5.3.1.8 Post-operative Treatment:
5.3.1.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist, and
using therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative. In all cases, communication between the physician and therapist
is important to the timing of weight-bearing, and exercise progressions.
5.3.1.8.2 Treatment usually includes active
therapy with or without passive therapy for early range of motion and
weight-bearing then progression to, strengthening, flexibility, neuromuscular
training, and gait training with appropriate assistive devices.
5.3.1.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.3.2 Aggravated Osteoarthritis:
5.3.2.1 Description/Definition:
hip pain with radiographic evidence of joint space narrowing or femoral
acetabular osteophytes, and sedimentation rate less than 20mm/hr with
symptoms. Patients usually have gradual
onset of pain increasing with use and relieved with rest, progressing to
morning stiffness and then to night pain.
5.3.2.2 Other
causative factors to consider: Prior significant injury to the hip may
predispose the joint to osteoarthritis.
In order to entertain previous trauma as a cause, the patient should
have a medically documented injury with radiographs or MRI showing the level of
anatomic change. The prior injury should
have been at least 2 years from the presentation for the new complaints and
there should be a significant increase of pathology on the affected side in
comparison to the original imaging or operative reports and/or the opposite
un-injured side or extremity.
5.3.2.3 Specific
Physical Exam Findings: Bilateral
exam including knees and low back is necessary to rule out other
diagnoses. Pain with the hip in external
and/or internal hip rotation with the knee in extension is the strongest
indicator.
5.3.2.4 Diagnostic
Testing Procedures: standing pelvic radiographs demonstrating joint space
narrowing to 2 mm or less, osteophytes or sclerosis at the joint. MRI may be ordered to rule out other more
serious disease.
5.3.2.5 Non-operative Treatment Procedures:
5.3.2.5.1 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
Medications and Medical Management.
5.3.2.5.2 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management. Patient education may also include videos,
telephone, follow-up, and pamphlets.
5.3.2.5.3 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. They should
include range-of-motion (ROM), active therapies and a home exercise
program. Active therapies include gait
training with appropriate assistive devices, proprioception training restoring
normal joint mechanics, and clearing dysfunctions from adjacent
structures. Passive as well as active
therapies may be used for control of pain and swelling. Therapy should progress
to strengthening and an independent home exercise program targeted to further
improve ROM, strength, and normal joint mechanics influenced by proximal and
distal structures. Therapy should
include training on the use of adaptive equipment and home and work site
evaluations when appropriate Refer to Section 6.0, Therapeutic Procedures,
Non-operative. There is good evidence
that a supervised therapeutic exercise program with an element of strengthening
is an effective treatment for hip osteoarthritis.
5.3.2.5.3.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active treatment.
They may be used as found as adjunctive in Section 6.0, Therapeutic Procedures,
Non-operative. There is some evidence
that manual therapy, including stretching and traction manipulation by a
trained provider, produces functional improvement in hip osteoarthritis and may
be a suitable treatment option.
5.3.2.5.3.1.1 Aquatic therapy may be used as
a type of active intervention to improve muscle strength and range of motion
when land-based therapy is not well-tolerated.
5.3.2.5.3.1.2 The use of insoles, adaptive
equipment, cane, may be beneficial.
5.3.2.5.3.1.3 There
is some evidence that acupuncture may produce improvement in hip pain and
function, making it a suitable treatment option for patients. Refer to Section 6.0,
Therapeutic Procedures, Non-operative.
5.3.2.5.4 Steroid Injections - Steroid
injections may decrease inflammation and allow the therapist to progress with
functional exercise and ROM.
5.3.2.5.4.1 Time to Produce Effect: One injection.
5.3.2.5.4.2 Maximum Duration: 3 injections in
one year spaced at least 4 to 8 weeks apart.
5.3.2.5.4.3 Steroid injections should be used
cautiously in diabetic patients. Diabetic patients should be reminded to check
their blood glucose levels at least daily for 2 weeks after injections. Injections may be performed with or without
ultrasound guidance. Ultrasound guided interventional procedure provides the
ability to image soft tissues in real time and can improve safety and accuracy
of needle placement. The use of
ultrasound guided procedures will be at the discretion of the health care
provider.
5.3.2.5.5 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.3.2.5.6 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.3.2.6 Surgical Indications/Considerations:
5.3.2.6.1 When pain interferes with ADLs and
the patient meets the following: 1) low surgical risk, 2) adequate bone
quality, and 3) failure of previous non-surgical interventions including weight
control, therapy with active patient participation, and medication. Refer to
Section 7.0, Therapeutic Procedures-operative, Hip Arthroplasty, for indications
specific to the procedure.
5.3.2.6.2 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.3.2.6.3 In cases where surgery is
contraindicated due to obesity, it may be appropriate to recommend a weight
loss program if the patient is unsuccessful losing weight on their own.
Coverage for weight loss would continue only for motivated patients who have
demonstrated continual progress with weight loss.
5.3.2.6.4 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that carriers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
5.3.2.7 Operative
Procedures: Prosthetic replacement
(traditional or minimally invasive), or resurfacing.
5.3.2.8 Post-operative Treatment:
5.3.2.8.1 In all cases, communication between
the physician and therapist is important to the timing of weight-bearing and
exercise progressions.
5.3.2.8.2 For prosthetic
replacement, refer to Section 7.0, Hip Arthroplasty.
5.3.2.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.3.3 Femoral
Osteonecrosis (Avascular Necrosis (AVN) of the Femoral Head):
5.3.3.1 Description/Definition: Death of the bone tissue of the femoral head
following loss of blood supply to the area. Destruction of the articular
surfaces of the hip joint may lead to arthritis.
5.3.3.2 Occupational
Relationship: Usually, from trauma
resulting in displaced subcapital fracture of the hip or hip dislocation may
cause AVN. Previous surgical procedures and systemic steroids may lead to AVN.
In the general population risk factors include, but are not limited to alcohol
abuse, smoking, Caisson disease (also known as the bends), sickle cell anemia,
autoimmune disease, and hypercoagulable states. Often, the cause cannot be
identified. Involvement of the opposite
hip may occur in more than half of cases not caused by trauma.
5.3.3.3 Specific
Physical Exam Findings: Hip or groin pain made worse by motion or
weight-bearing and alleviated by rest is the classical presentation. Symptoms
may begin gradually, often months after the vascular compromise of blood flow.
A limp may result from the limited toleration of weight-bearing.
5.3.3.4 Diagnostic
Testing Procedures: X-ray
abnormalities include sclerotic changes, cystic lesions, joint space narrowing,
and degeneration of the acetabulum. The x-ray may be normal in the first
several months of the disease process. AVN should be suspected when hip pain
occurs and risk factors are present. X-rays should be done first, but may be
followed by an MRI. When AVN is not due
to trauma, both hips should be imaged.
5.3.3.5 Non-operative Treatment Procedures:
5.3.3.5.1 Initial Treatment: protected weight-bearing and bracing followed
by active therapy with or without passive therapy. Conservative approaches may suffice when the
lesion is small, but larger lesions are expected to require surgical
intervention when symptoms are disabling.
5.3.3.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
Medications and Medical Management.
5.3.3.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management. Weight-bearing restrictions
may be appropriate.
5.3.3.5.4 Smoking may affect bone healing.
Patients should be strongly encouraged to stop smoking and be provided with
appropriate counseling by the physician.
5.3.3.5.5 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to Section 6.0, Return to Work.
5.3.3.5.6 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative, may be employed in individual cases.
5.3.3.6 Surgical
Indications/Considerations: Core
decompression may appropriate for some patients with early disease (Stages 1
and 2A) who have functionally disabling symptoms. Femoral head osteotomies or
resurfacing hemiarthroplasties may also be appropriate for younger patients
when disease is limited to the femoral head.
Those 50 or older and patients with total joint collapse or severely
limiting disease will usually require an implant arthroplasty.
5.3.3.6.1 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.3.3.6.2 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that insurers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
5.3.3.7 Operative
Procedures: Osteotomy, core decompression with or without bone graft,
prosthetic replacement. Refer to Section 7.0, Therapeutic Procedures-operative
for details.
5.3.3.8 Post-operative Treatment:
5.3.3.8.1 Anticoagulant therapy to prevent deep
venous thrombosis for most procedures. Refer Section 6.0, Therapeutic
Procedures, Non-operative.
5.3.3.8.2 Treatment usually includes active
therapy with or without passive therapy.
Refer to Section 7.0 and specific procedures for further details.
5.3.3.8.3 An individualized rehabilitation
program based upon communication between the surgeon and the therapist using
the treatments found in Section 6.0, Therapeutic Procedures, Non-operative.
5.3.3.8.4 Treatment should include
gait training with appropriate assistive devices.
5.3.3.8.5 Therapy should include training on
the use of adaptive equipment and home and work site evaluation when
appropriate.
5.3.3.8.6 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon
5.3.4 Femur Fracture:
5.3.4.1 Description/Definition: Fracture of the femur distal to the lesser
trochanter.
5.3.4.2 Occupational Relationship: Usually from a traumatic injury such as
a fall or crush.
5.3.4.3 Specific
Physical Exam Findings: May have a short, abnormally rotated
extremity. Effusion if the knee joint is
involved.
5.3.4.4 Diagnostic
Testing Procedures:
Radiographs. Occasionally CT scan
or MRI, particularly if the knee joint is involved.
5.3.4.5 Non-operative Treatment Procedures:
5.3.4.5.1 Initial Treatment: Although surgery is usually required, non- operative procedures may be considered
in stable, non- displaced fractures
and will require protected weight-bearing.
5.3.4.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
Medications and Medical Management.
5.3.4.5.3 Back pain may occur after femur
fracture and should be addressed and treated as necessary.
5.3.4.5.4 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, weight management. Weight-bearing restrictions may
be appropriate.
5.3.4.5.5 Refer
to comments related to osteoporosis in Section 6.0, Therapeutic
Procedures, Non-operative, under the subsection for Osteoporosis Management.
5.3.4.5.6 Smoking may affect fracture healing.
Patients should be strongly encouraged to stop smoking and be provided with
appropriate counseling by the physician.
5.3.4.5.7 Orthotics such as heel lifts and
custom shoe build-ups may be required when leg-length discrepancy persists.
5.3.4.5.8 Return to work with appropriate restrictions
should be considered early in the course of treatment. Refer to Section 6.0, under
the subsection for Return to Work.
5.3.4.5.9 Other therapies in Section 6.0, Therapeutic
Procedures, Non-Operative may be employed in individual cases.
5.3.4.6 Surgical
Indications/Considerations: Femoral
neck fracture or supracondylar femur fracture with joint incongruity.
5.3.4.6.1 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that insurers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
5.3.4.7 Operative Procedures: Rod
placement or open internal fixation.
5.3.4.8 Post-operative Treatment:
5.3.4.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist, using
therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative. In all cases,
communication between the physician and the therapist is important to the
timing of weight-bearing and exercise progression.
5.3.4.8.2 Treatment usually includes active
therapy with or without passive therapy for protected weight-bearing, early
range of motion if joint involvement.
5.3.4.8.3 Refer to bone-growth stimulators in
Section 6.0, Therapeutic Procedures, Non-operative.
5.3.4.8.4 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.3.5 Hamstring Tendon Rupture:
5.3.5.1 Description/Definition:
Most commonly, a disruption of the muscular portion of the hamstring. Extent of the tear is variable. Occasionally a proximal tear or
avulsion. Rarely a distal injury.
5.3.5.2 Occupational
Relationship: Usually from excessive tension on the hamstring either from
an injury or from a rapid, forceful contraction of the muscle.
5.3.5.3 Specific Physical Exam Findings: Local tenderness, swelling,
ecchymosis.
5.3.5.4 Diagnostic
Testing Procedures: Occasionally
radiographs, musculoskeletal ultrasound, or MRI for proximal tears/possible
avulsion.
5.3.5.5 Non-operative Treatment Procedures:
5.3.5.5.1 Initial Treatment:
Protected weight-bearing and ice.
5.3.5.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
in the Medications and Medical Management subsection.
5.3.5.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, and weight management.
5.3.5.5.4 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. They may include
range-of-motion (ROM), active therapies, and a home exercise program. Active therapies include proprioception
training, restoring normal joint mechanics, and clearing dysfunctions from
adjacent structures. Passive as well as active therapies may be used for
control of pain and swelling. Therapy should progress to strengthening and an
independent home exercise program targeted to further improve ROM, strength,
and normal joint mechanics influenced by proximal and distal structures.
Bracing may be appropriate. Refer to Section 6.0, Therapeutic Procedures,
Non-operative.
5.3.5.5.4.1 Passive modalities are most effective
as adjunctive treatments to improve the results of active treatment. They may
be used as found as adjunctive in Section 6.0, Therapeutic Procedures,
Non-operative.
5.3.5.5.5 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.3.5.5.6 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.3.5.6 Surgical Indications/Considerations:
5.3.5.6.1 Surgery is indicated for proximal or
distal injuries only when significant functional impairment is expected without
repair. If surgery is indicated, it is preferably performed within three
months.
5.3.5.6.2 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.3.5.6.3 Smoking may affect soft tissue healing
through tissue hypoxia. Patients should be strongly encouraged to stop smoking
and be provided with appropriate counseling by the physician.
5.3.5.7 Operative
Procedures: Re-attachment of
proximal avulsions and repair of distal tendon disruption.
5.3.5.8 Post-operative Treatment:
5.3.5.8.1 An individualized rehabilitation
program based upon communication between the surgeon/physician and the therapy provider using therapies as outlined
in Section 6.0, Therapeutic Procedures, Non-operative. In all cases, communication between the
physician and therapist is important to the timing of weight-bearing and
exercise progressions.
5.3.5.8.2 Treatment may include protected
weight-bearing and active therapy with or without passive therapy. Splinting in a functional brace may reduce
time off work.
5.3.5.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.3.6 Hip Dislocation:
5.3.6.1 Description/Definition: Disengagement of the femoral head from the
acetabulum.
5.3.6.2 Occupational Relationship: Usually from a traumatic injury such as
a fall or crush.
5.3.6.3 Specific
Physical Exam Findings: Most commonly a short, internally rotated, adducted
lower extremity with a posterior dislocation and a short externally rotated
extremity with an anterior dislocation.
5.3.6.4 Diagnostic Testing Procedures: Radiographs, CT scanning.
5.3.6.5 Non-operative Treatment Procedures:
5.3.6.5.1 Initial Treatment: Urgent closed reduction with sedation or general anesthesia.
5.3.6.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
the Medications and Medical Management subsection.
5.3.6.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.3.6.5.4 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. They should
include bracing then range-of-motion (ROM), active therapies, and a home
exercise program. Active therapies
include proprioception training, gait training with appropriate assistive
devices, restoring normal joint mechanics, and clearing dysfunctions from
adjacent structures. Passive as well as active therapies may be used for
control of pain and swelling. Therapy should progress to strengthening and an
independent home exercise program targeted to further improve ROM, strength,
and normal joint mechanics influenced by proximal and distal structures.
Therapy should include training on the use of adaptive equipment and home and
work site evaluations when appropriate. Bracing may be appropriate Refer to
Section 6.0, Therapeutic Procedures, Non-operative.
5.3.6.5.4.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active treatment.
They may be used as found as adjunctive in Section 6.0, Therapeutic Procedures,
Non-operative.
5.3.6.5.5 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.3.6.5.6 Other therapies in Section 6.0, Therapeutic
Procedures, Non-operative may be employed in individual cases.
5.3.6.6 Surgical
Indications/Considerations: Failure of closed reduction. Associated fracture of the acetabulum or
femoral head, loose fragments in joint or open fracture.
5.3.6.6.1 Because smokers have a higher risk of
non-union and post-operative costs, when a fracture is involved it is
recommended that insurers cover a smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as cotinine
levels for long-term cessation.
5.3.6.7 Operative
Procedures: Open reduction of the femoral head or acetabulum and possible
internal fixation.
5.3.6.8 Post-operative Treatment Procedures:
5.3.6.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist using
therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative. In all cases,
communication between the physician and therapist is important to the timing of
weight-bearing and exercise progressions.
5.3.6.8.2 Treatment
should include gait training with appropriate assistive devices.
5.3.6.8.3 Treatment
may include protected weight-bearing and active therapy with or without passive
therapy for early range of motion.
5.3.6.8.4 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.3.7 Hip Fracture:
5.3.7.1 Description/Definition: Fractures of the neck and peri-trochanteric
regions of the proximal femur.
5.3.7.2 Occupational
Relationship: Usually from a traumatic injury such as a fall or crush. Patients with intracapsular femoral fractures
have a risk of developing avascular necrosis of the femoral head requiring
treatment months to years after the initial injury.
5.3.7.3 Specific Physical Exam Findings: Often a short and externally
rotated lower extremity.
5.3.7.4 Diagnostic Testing Procedures:
Radiographs. Occasional use of CT
scan or MRI.
5.3.7.5 Non-operative Treatment Procedures:
5.3.7.5.1 Initial Treatment: protected weight-bearing and bracing followed
by active therapy with or without passive therapy. Although surgery is usually required,
non-operative procedures may be considered in stable, non-displaced fractures.
5.3.7.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section
6.0, the Medications and Medical Management subsection.
5.3.7.5.3 Back pain may occur after hip
fracture and should be addressed and treated as necessary.
5.3.7.5.4 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management. Weight-bearing restrictions
may be appropriate.
5.3.7.5.5 Refer to comments on
osteoporosis in Section 5.0, Ankle Sprain/Fracture.
5.3.7.5.6 Smoking may affect fracture healing.
Patients should be strongly encouraged to stop smoking and be provided with
appropriate counseling by the physician.
5.3.7.5.7 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to Section 6.0, Return to Work.
5.3.7.5.8 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative, may be employed in individual cases.
5.3.7.6 Surgical
Indications/Considerations: Surgery
is indicated for unstable peritrochanteric fractures and femoral neck
fractures.
5.3.7.6.1 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that insurers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
5.3.7.7 Operative
Procedures: Prosthetic replacement
for displaced femoral neck fractures. Reduction and internal fixation for
peritrochanteric fractures, and un-displaced, or minimally-displaced neck
fractures.
5.3.7.8 Post-operative Treatment:
5.3.7.8.1 Anti coagulant therapy to prevent
deep venous thrombosis. Refer Section 6.0, Therapeutic Procedures,
Non-operative.
5.3.7.8.2 Treatment usually
includes active therapy with or without passive therapy.
5.3.7.8.3 An individualized rehabilitation
program based upon communication between the surgeon and the therapist using
the treatments found in Section 6.0, Therapeutic Procedures, Non-operative.
5.3.7.8.4 Treatment should include
gait training with appropriate assistive devices.
5.3.7.8.5 Therapy should include training on
the use of adaptive equipment and home and work site evaluation when
appropriate.
5.3.7.8.6 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.3.8 Impingement/Labral
Tears:
5.3.8.1 Description/Definition:
Two types of impingement are described pincer; resulting from over coverage of
the acetabulum and/or cam; resulting from aspherical portion of the head and
neck junction. Persistence of these abnormalities can cause early arthritis or
labral tears. Labral tears can also be
isolated; however, they are frequently accompanied by bony abnormalities. Patients usually complain of catching or
painful clicking which should be distinguished from a snapping iliopsoas tibial
tendon. A pinch while sitting may be reported and hip or groin pain.
5.3.8.2 Occupational
Relationship: Impingement abnormalities are usually congenital; however, they
may be aggravated by repetitive rotational force or trauma. Labral tears may accompany impingement or
result from high energy trauma.
5.3.8.3 Specific Physical Exam Findings:
Positive labral tests.
5.3.8.4 Diagnostic
Testing Procedures: Cross table laterals, standing AP pelvis and frog leg lateral x-rays. MRI
may reveal abnormality; however, false positives and false negatives are also
possible. MRI
arthrogram with gadolinium should be performed to diagnose labral tears, not a
pelvic MRI. Intra-articular injection should help rule
out extra-articular pain generators. To
confirm the diagnosis, the
patient should demonstrate changes on a pain scale accompanied by recorded
functional improvement post-injection. This is important, as labral tears do
not always cause pain and over-diagnosis is possible using imaging alone. Injections may be performed with or without
ultrasound guidance. Ultrasound guided interventional procedure provides the
ability to image soft tissues in real time and can improve safety and accuracy
of needle placement. The use of
ultrasound guided procedures will be at the discretion of the health care
provider.
5.3.8.5 Non-operative Treatment Procedures:
5.3.8.5.1 Medications
such as analgesics and anti-inflammatories may be helpful. Refer to medication
discussions in Section 6.0, Medications and Medical Management.
5.3.8.5.2 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, reducing
hip adduction and internal rotation home exercise, joint protection, and weight management.
5.3.8.5.3 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions. They should
include range-of-motion (ROM), active therapies and a home exercise
program. Active therapies include
proprioception training, restoring normal joint mechanics, and clearing
dysfunctions from adjacent structures.
Passive as well as active therapies may be used for control of pain and
swelling. Therapy should progress to strengthening and an independent home
exercise program targeted to further improve ROM, strength, and normal joint
mechanics influenced by proximal and distal structures. Refer to Section 6.0,
Therapeutic Procedures, Non-operative.
5.3.8.5.3.1 Passive
modalities are most effective as adjunctive treatments to improve the results
of active treatment. They may be used as found as adjunctive in Section 6.0,
Therapeutic Procedures, Non-operative.
5.3.8.5.4 Steroid
Injections - Steroid injections may decrease inflammation and allow the
therapist to progress with functional exercise and ROM.
5.3.8.5.4.1 Time to Produce Effect: One
injection.
5.3.8.5.4.2 Maximum Duration: 3 injections in
one year spaced at least 4 to 8 weeks apart.
5.3.8.5.4.3 Steroid injections should be used
cautiously in diabetic patients. Diabetic patients should be reminded to check
their blood glucose levels at least daily for 2 weeks after injections. Injections may be performed with or without
ultrasound guidance. Ultrasound guided interventional procedure provides the
ability to image soft tissues in real time and can improve safety and accuracy
of needle placement. The use of
ultrasound guided procedures will be at the discretion of the health care
provider.
5.3.8.5.5 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.3.8.5.6 Other
therapies in Section 6.0, Therapeutic Procedures, Non-operative may be employed
in individual cases.
5.3.8.6 Surgical Indications/Considerations:
5.3.8.6.1 Surgery is indicated when 1)
functional limitations persist after 8 weeks of active patient participation in
treatment, 2) there are clinical signs and symptoms suggestive of the diagnosis
and 3) other diagnoses have been ruled out.
5.3.8.6.2 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
5.3.8.6.3 In cases where surgery is
contraindicated due to obesity, it may be appropriate to recommend a weight
loss program if the patient is unsuccessful losing weight on their own.
Coverage for weight loss would continue only for motivated patients who have
demonstrated continual progress with weight loss.
5.3.8.6.4 Smoking may affect soft tissue
healing through tissue hypoxia. Patients should be strongly encouraged to stop
smoking and be provided with appropriate counseling by the physician.
5.3.8.7 Operative Procedures: Debridement or repair of labrum and removal
of excessive bone.
5.3.8.8 Post-operative Treatment:
5.3.8.8.1 When bone is removed and/or the
labrum is repaired, weight-bearing restrictions usually apply.
5.3.8.8.2 An individualized rehabilitation
program based upon communication between the surgeon and the therapist that
should include gait training with appropriate assistive devices. Refer to
Section 6.0, Therapeutic Procedures Non-operative.
5.3.8.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.3.9 Pelvic Fracture:
5.3.9.1 Description/Definition:
Fracture of one or more components of the pelvic ring (sacrum and iliac wings).
5.3.9.2 Occupational Relationship: Usually from a traumatic injury such as
a fall or crush.
5.3.9.3 Specific
Physical Exam Findings: Displaced fractures may cause pelvic deformity and
shortening, or rotation of the lower extremities.
5.3.9.4 Diagnostic
Testing Procedures: Radiographs, CT scanning. Occasionally MRI, angiogram, urethrogram,
emergent sonogram.
5.3.9.5 Non-operative Treatment Procedures:
5.3.9.5.1 Initial Treatment: Protected weight-bearing. Although surgery is usually required,
non-operative procedures may be considered in a stable, non-displaced fracture.
5.3.9.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
the Medications and Medical Management subsection.
5.3.9.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.3.9.5.4 Refer to comments related to
osteoporosis in Section 6.0, Therapeutic Procedures, Non-operative,
Osteoporosis Management.
5.3.9.5.5 Smoking may affect fracture healing.
Patients should be strongly encouraged to stop smoking and be provided with
appropriate counseling by the physician.
5.3.9.5.6 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions, after boney union
has been achieved. They should include bracing then range-of-motion (ROM),
active therapies, and a home exercise program.
Active therapies include, proprioception training, gait training with
appropriate assistive devices, restoring normal joint mechanics, and clearing
dysfunctions from adjacent structures. Passive as well as active therapies may
be used for control of pain and swelling. Therapy should progress to
strengthening and an independent home exercise program targeted to further
improve ROM, strength, and normal joint mechanics influenced by proximal and
distal structures. Therapy should
include training on the use of adaptive equipment and home and work site
evaluations when appropriate. Refer to Section 6.0, Therapeutic Procedures,
Non-operative.
5.3.9.5.6.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active treatment.
They may be used as found as adjunctive in Section 6.0, Therapeutic Procedures,
Non-operative.
5.3.9.5.7 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, the Return to Work subsection.
5.3.9.5.8 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.3.9.6 Surgical Indications/Considerations: Unstable fracture pattern, or
open fracture.
5.3.9.6.1 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that insurers cover a
smoking cessation program peri-operatively.
Physicians may monitor
smoking cessation with laboratory tests such as cotinine levels for long-term
cessation.
5.3.9.7 Operative Procedures: External or internal fixation dictated by
fracture pattern.
5.3.9.8 Post-operative Treatment:
5.3.9.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist using therapies
as outlined in Section 6.0, Therapeutic Procedures, Non-operative. In all cases, communication between the
physician and therapist is important to the timing of weight-bearing and
exercise progressions.
5.3.9.8.2 Treatment usually includes active
therapy with or without passive therapy for gait, pelvic stability,
strengthening, and restoration of joint and extremity function. Treatment should include gait training with
appropriate assistive devices.
5.3.9.8.3 Graduated weight-bearing
according to fracture healing.
5.3.9.8.4 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.3.10 Tendonopathy: Refer to Tendonopathy in
Section 5.0 for general recommendations.
5.3.11 Tibial
Fracture:
5.3.11.1 Description/Definition: Fracture of the tibia proximal to the
malleoli.
Open
tibial fractures are graded in severity according to the Gustilo-Anderson
Classification:
5.3.11.1.1 Type I: Less than 1 cm (puncture wounds).
5.3.11.1.2 Type II: 1 to 10 cm.
5.3.11.1.3 Type III-A: Greater than 10 cm, sufficient soft
tissue preserved to cover the wound (includes gunshot wounds and any injury in
a contaminated environment).
5.3.11.1.4 Type III-B: Greater than 10 cm,
requiring a soft tissue coverage procedure.
5.3.11.1.5 Type III-C: With vascular injury
requiring repair.
5.3.11.2 Occupational Relationship: Usually from a traumatic injury such as
a fall or crush.
5.3.11.3 Specific Physical Exam Findings: May have a short, abnormally
rotated extremity. Effusion if the knee
joint involved.
5.3.11.4 Diagnostic Testing Procedures:
Radiographs. CT scanning or MRI.
5.3.11.5 Non-operative Treatment Procedures:
5.3.11.5.1 Initial Treatment: Protected weight-bearing; functional
bracing. There is some evidence for use
of pneumatic braces with stress fractures.
5.3.11.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section
6.0, Medications and Medical Management.
5.3.11.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.3.11.5.4 Refer to comments related to
osteoporosis in Section 6.0, Therapeutic Procedures, Non-operative, Osteoporosis
Management.
5.3.11.5.5 Smoking may affect fracture healing.
Patients should be strongly encouraged to stop smoking and be provided with
appropriate counseling by the physician.
5.3.11.5.6 Benefits may be achieved through
therapeutic rehabilitation and rehabilitation interventions, after boney union
has been achieved. They should include bracing then range-of-motion (ROM),
active therapies including proprioception training, restoring normal joint
mechanics, and clearing dysfunctions from adjacent structures, and a home
exercise program. Passive as well as active therapies may be used for control
of pain and swelling. Therapy should progress to strengthening and an
independent home exercise program targeted to further improve ROM, strength,
restoring normal joint mechanics, influenced by proximal and distal structures.
Therapy should include training on the use of adaptive equipment and home and
work site evaluations when appropriate.
Bracing may be appropriate. Refer to Section 6.0, Therapeutic Procedures,
Non-operative.
5.3.11.5.6.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active treatment.
They may be used as found as adjunctive in Section 6.0, Therapeutic Procedures,
Non-operative.
5.3.11.5.7 Orthotics such as heel lifts and
custom shoe build-ups may be required when leg-length discrepancy persists.
5.3.11.5.8 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, the Return to Work
subsection.
5.3.11.5.9 Other therapies in Section 6.0,
Therapeutic Procedures, Non-Operative may be employed in individual cases.
5.3.11.6 Surgical Indications/Considerations: Unstable fracture pattern,
displaced fracture (especially if the knee joint is involved), open fracture,
and non-union.
5.3.11.6.1 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that insurers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
5.3.11.7 Operative Procedures: Often closed rodding for shaft
fractures. Open reduction and internal
fixation more common for fractures involving the knee joint or pilon fractures of
the distal tibia.
5.3.11.7.1 Human
bone morphogenetic protein (RhBMP): this material is used for surgical repair
of open tibial fractures. Refer to
Section 7.0, Therapeutic Procedures, Operative for further specific
information.
5.3.11.7.2 Stem cell use - stem cells have been
added to allograft to increase fracture union.
Their use is considered experimental and is not recommended at this
time.
5.3.11.8 Post-operative Treatment:
5.3.11.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist and
using therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative. In all cases,
communication between the physician and therapist is important to the timing of
weight-bearing and exercise progressions.
5.3.11.8.2 Treatment may include protected
weight-bearing and active therapy with or without passive therapy for early
range of motion if joint involvement.
5.3.11.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
5.3.12 Trochanteric
Fracture:
5.3.12.1 Description/Definition: Fracture of the greater trochanter of the
proximal femur.
5.3.12.2 Occupational Relationship: Usually from a traumatic injury such as
a fall or crush.
5.3.12.3 Specific Physical Exam Findings: Local tenderness over the greater
trochanter. Sometimes associated swelling, ecchymosis.
5.3.12.4 Diagnostic Testing Procedures: Radiographs, CT scans or MRI.
5.3.12.5 Non-operative Treatment Procedures:
5.3.12.5.1 Initial Treatment:
protected weight-bearing.
5.3.12.5.2 Medications such as analgesics and
anti-inflammatories may be helpful. Refer to medication discussions in Section 6.0,
Medications and Medical Management.
5.3.12.5.3 Patient education should include
instruction in self-management techniques, ergonomics, body mechanics, home
exercise, joint protection, and weight management.
5.3.12.5.4 Refer to comments related to
osteoporosis in Section 6.0, Therapeutic Procedures, Non-operative,
Osteoporosis Management.
5.3.12.5.5 Smoking may affect fracture healing.
Patients should be strongly encouraged to stop smoking and be provided with
appropriate counseling by the physician.
5.3.12.5.6 Benefits may be achieved through therapeutic
rehabilitation and rehabilitation interventions, after boney union has been
achieved. They should include bracing then range-of-motion (ROM), active
therapies, and a home exercise program.
Active therapies include proprioception training, restoring normal joint
mechanics, and clearing dysfunctions from adjacent structures, and a home
exercise program. Passive as well as active therapies may be used for control
of pain and swelling. Therapy should progress to strengthening and an
independent home exercise program targeted to further improve ROM, strength,
and normal joint mechanics influenced by proximal and distal structures.
Bracing may be appropriate. Refer to Section 6.0, Therapeutic Procedures,
Non-operative.
5.3.12.5.6.1 Passive modalities are most
effective as adjunctive treatments to improve the results of active treatment.
They may be used as found as adjunctive in Section 6.0, Therapeutic Procedures,
Non-operative.
5.3.12.5.7 Return to work with appropriate
restrictions should be considered early in the course of treatment. Refer to
Section 6.0, Return to Work.
5.3.12.5.8 Other therapies in Section 6.0,
Therapeutic Procedures, Non-operative may be employed in individual cases.
5.3.12.6 Surgical Indications/Considerations: Large, displaced fragment,
open fracture.
5.3.12.6.1 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that insurers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
5.3.12.7 Operative Procedures: Open reduction, internal fixation.
5.3.12.8 Post-operative Treatment:
5.3.12.8.1 An individualized rehabilitation
program based upon communication between the surgeon and the therapist using
therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative. In all cases,
communication between the physician and therapist is important to the timing of
weight-bearing and exercise progressions.
5.3.12.8.2 Protected weight-bearing is usually
needed. Full weight-bearing with radiographic and clinical signs of healing.
5.3.12.8.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
|
|
Treating
providers, as well as employers and insurers are highly encouraged to reference
the General Guidelines Principles (Section 2.0) prior to initiation of any
therapeutic procedure. Before initiation
of any therapeutic procedure, the authorized treating provider, employer and
insurer must consider these important issues in the care of the injured worker.
First, patients
undergoing therapeutic procedure(s) should be released or returned to modified,
restricted duty during their rehabilitation at the earliest appropriate
time. Refer to Return-to-Work in this
section for detailed information.
Second,
cessation and/or review of treatment modalities should be undertaken when no
further significant subjective or objective improvement in the patient’s
condition is noted. If patients are not
responding within the recommended duration periods, alternative treatment
interventions, further diagnostic studies or consultations should be pursued.
Third, providers
should provide and document education to the patient. No treatment plan is complete without
addressing issues of individual and/or group patient education as a means of
facilitating self-management of symptoms.
Last, formal
psychological or psychosocial evaluation should be performed on patients not
making expected progress within 6 to 12 weeks following injury and whose
subjective symptoms do not correlate with objective signs and tests. Earlier psychological intervention may be
necessary and will be dependent on the treating physician’s assessment of
psychological or psychosocial issues that may interfere with the patient’s
recovery.
In cases where a
patient is unable to attend an outpatient center, home therapy may be
necessary. Home therapy may include active
and passive therapeutic procedures as well as other modalities to assist in
alleviating pain, swelling, and abnormal muscle tone. Home therapy is usually of short duration and
continues until the patient is able to tolerate coming to an outpatient center.
The following
procedures are listed in alphabetical order.
6.1 ACUPUNCTURE
is an accepted and widely used procedure for the relief of pain and inflammation. The exact mode of action is only partially understood. Western medicine studies suggest that acupuncture stimulates the nervous system at the level of the brain, promotes deep relaxation, and affects the release of neurotransmitters. Acupuncture is commonly used as an alternative or in addition to traditional Western pharmaceuticals. While it is commonly used when pain medication is reduced or not tolerated, it may be used as an adjunct to physical rehabilitation and/or surgical intervention to hasten the return of functional activity. Acupuncture should be performed by MD, DO, DC with appropriate training; or a licensed acupuncturist.
6.1.1 Acupuncture: is the insertion
and removal of filiform needles to stimulate acupoints (acupuncture
points). Needles may be inserted,
manipulated and retained for a period of time. Acupuncture can be used to
reduce pain, reduce inflammation, increase blood flow, increase
range-of-motion, decrease the side effect of medication-induced nausea, promote
relaxation in an anxious patient, and reduce muscle spasm.
6.1.1.1 Indications
include joint pain, joint stiffness, soft tissue pain and inflammation,
paresthesia, post-surgical pain relief, muscle spasm, and scar tissue pain.
6.1.2 Acupuncture with Electrical
Stimulation: is
the use of electrical current (micro-amperage or milli-amperage) on the needles
at the acupuncture site. It is used to
increase effectiveness of the needles by continuous stimulation of the
acupoint. Physiological effects (depending
on location and settings) can include endorphin release for pain relief,
reduction of inflammation, increased blood circulation, analgesia through
interruption of pain stimulus, and muscle relaxation.
6.1.2.1 It is indicated to treat chronic pain
conditions, radiating pain along a nerve pathway, muscle spasm, inflammation,
scar tissue pain, and pain located in multiple sites.
6.1.3 Total Time Frames for Acupuncture and Acupuncture
with Electrical Stimulation: Time frames are not meant to be
applied to each of the above sections separately. The time frames are to be
applied to all acupuncture treatments regardless of the type or combination of
therapies being provided.
6.1.3.1 Time to Produce Effect: 3
to 6 treatments.
6.1.3.2 Frequency: 1 to 3 times per
week.
6.1.3.3 Optimum Duration: 1 to 2
months.
6.1.3.4 Maximum Duration: 14
treatments.
6.1.3.5 Any of the above acupuncture treatments
may extend longer if objective functional gains can be documented or when
symptomatic benefits facilitate progression in the patient’s treatment
program. Treatment beyond 14 treatments
must be documented with respect to need and ability to facilitate positive
symptomatic or functional gains. Such
care should be re-evaluated and documented with each series of treatments.
6.1.4 Other Acupuncture Modalities: Acupuncture treatment is based on individual
patient needs and therefore treatment may include a combination of procedures
to enhance treatment effect. Other
procedures may include the use of heat, soft tissue manipulation/massage, and
exercise. Refer to Active Therapy
(Therapeutic Exercise) and Passive Therapy sections (Massage and Superficial
Heat and Cold Therapy) for a description of these adjunctive acupuncture
modalities and time frames.
6.2 BIOFEEDBACK is a form of behavioral medicine that helps patients
learn self-awareness and self-regulation skills for the purpose of gaining
greater control of their physiology, such as muscle activity, brain waves, and
measures of autonomic nervous system activity.
Electronic instrumentation is used to monitor the targeted physiology
and then displayed or fed back to the patient visually, auditorially, or
tactilely, with coaching by a biofeedback specialist. Biofeedback is provided by clinicians
certified in biofeedback and/or who have documented specialized education,
advanced training, or direct or supervised experience qualifying them to
provide the specialized treatment needed (e.g., surface EMG, EEG, or other).
6.2.1 Treatment is individualized to
the patient’s work-related diagnosis and needs.
Home practice of skills is required for mastery and may be facilitated
by the use of home training tapes. The
ultimate goal in biofeedback treatment is normalizing the physiology to the
pre-injury status to the extent possible and involves transfer of learned
skills to the workplace and daily life.
Candidates for biofeedback therapy or training must be motivated to
learn and practice biofeedback and self-regulation techniques.
6.2.2 Indications for biofeedback
include individuals who are suffering from musculoskeletal injury in which
muscle dysfunction or other physiological indicators of excessive or prolonged
stress response affects and/or delays recovery.
Other applications include training to improve self-management of
emotional stress/pain responses such as anxiety, depression, anger, sleep
disturbance, and other central and autonomic nervous system imbalances. Biofeedback is often utilized along with
other treatment modalities.
6.2.3 Time to Produce Effect: 3 to 4
sessions.
6.2.4 Frequency: 1 to 2 times per week.
6.2.5 Optimum Duration: 5 to 6 sessions.
6.2.6 Maximum Duration: 10 to 12 sessions. Treatment beyond 12 sessions must be
documented with respect to need, expectation, and ability to facilitate
functional gains.
6.3 BONE-GROWTH STIMULATORS
6.3.1 Electrical: Pre-clinical and experimental literature has shown a
stimulatory effect of externally applied electrical fields on the proliferation
and calcification of osteoblasts and periosteal cells. All of the studies on bone growth
stimulators, however, have some methodological deficiencies and high-quality
literature of electrical bone growth stimulation is lacking for lower extremity
injuries.
6.3.1.1 These acceptable nonsurgical techniques
include Capacitive Coupling (CC), which places skin electrodes on opposite
sides of the bone being treated and Pulsed Electromagnetic Field (PEMF) which
uses a current-carrying coil which induces a secondary electrical field in
bone.
6.3.2 Low-intensity
Pulsed Ultrasound: There
is some evidence that low-intensity pulsed ultrasound, applied by the patient
at home and administered as initial treatment of the fracture, reduces the time
required for cortical bridging in tibial fractures. Non-union and delayed unions were not
included in these clinical trials. Possible indications for Low-Intensity
Pulsed Ultrasound are non-unions or fractures that are expected to require
longer healing time.
6.3.2.1 FDA approved bone-growth stimulators of
any type may be appropriate for patients with non-union after initial fracture
care or for patients with acute fractures or osteotomies who are at high risk
for delayed union or non-union. Patients
at high risk include, but are not limited to, smokers, diabetics, and those on
chemotherapeutic agents or other long-term medication affecting bone
growth.
6.4 EXTRACORPOREAL SHOCK WAVE THERAPY (ESWT)
6.4.1 Extracorporeal shock wave
therapy (ESWT) delivers an externally applied acoustic pulse to the plantar
fascia. It has been hypothesized that ESWT causes microtrauma to the fascia,
inducing a repair process involving the formation of new blood vessels and
delivery of nutrients to the affected area. High energy ESWT is delivered in
one session and may be painful requiring some form of anesthesia. It is not
generally recommended for the treatment of plantar heel pain due to increased
cost when it is performed with conscious sedation. It may also be performed with local blocks.
Low energy ESWT does not require anesthetics.
It is given in a series of treatments, generally three sessions.
6.4.2 There is conflicting evidence
concerning low energy ESWT for plantar heel pain. Focused ESWT concentrates the
acoustic pulse on a single point in the heel, while radial ESWT distributes the
pulse along the entire plantar fascia. Focused low energy ESWT has not been
shown to produce clinically important reductions in plantar heel pain. There is
some evidence that radial ESWT may reduce plantar pain more effectively than
placebo, but a successful response may occur in only 60% of patients. There is some evidence supporting high-energy
ESWT.
6.4.3 Low energy radial or high
energy ESWT with local blocks are accepted treatments. It should only be used
on patients who have had plantar pain for 4 months or more; have tried NSAIDs,
ice, stretching exercises, shoe inserts; and have significant functional
deficits. These patients should meet the
indications for surgery found in Section 5.0, heel spurs, plantar fascia
pain. Tarsal tunnel syndrome should be
ruled out. Peripheral vascular disease,
lower extremity neuropathy and diabetes are all relative
contraindications. Diagnostic testing
may be needed to rule out these conditions.
6.4.4 Time to Effect: 2 sessions.
6.4.5 Optimum Duration: 3 sessions
one week or more apart.
6.4.6 Maximum Duration: Treatment may
be continued for up to 5 total sessions if functional improvement has been
demonstrated after three treatment sessions. Functional improvement is
preferably demonstrated using direct testing or functional scales validated in
clinical research settings.
6.5 INJECTIONS-THERAPEUTIC
6.5.1 General Description: Therapeutic
injection procedures may play a significant role in the treatment of patients
with lower extremity pain or pathology.
Therapeutic injections involve the delivery of anesthetic and/or
anti-inflammatory medications to the painful structure. Therapeutic injections have many potential
benefits. Ideally, a therapeutic
injection will: (a) reduce inflammation in a specific target area; (b) relieve
secondary muscle spasm; (c) allow a break from pain; and (d) support therapy
directed to functional recovery.
Diagnostic and therapeutic injections should be used early and
selectively to establish a diagnosis and support rehabilitation. If injections are overused or used outside
the context of a monitored rehabilitation program, they may be of significantly
less value. . All injections may be performed with or
without ultrasound guidance. Ultrasound
guided interventional procedures provides the ability to image soft tissues in
real time and can improve safety and accuracy of needle placement. The use of ultrasound guided procedures will
be at the discretion of the health care provider.
6.5.2 General Indications: Diagnostic
injections are procedures which may be used to identify pain generators or
pathology. For additional specific
clinical indications, see Section 5.0, Specific Lower Extremity Injury
Diagnosis, Testing and Treatment.
6.5.3 Special Considerations: The
use of injections has become progressively sophisticated. Each procedure considered has an inherent
risk, and risk versus benefit should be evaluated when considering injection
therapy. In addition, all injections
must include sterile technique.
6.5.4 General Contraindications: General contraindications include local or
systemic infection, bleeding disorders, allergy to medications used, and
patient refusal. Specific
contraindications may apply to individual injections.
6.5.5 Joint Injections: are generally
accepted, well-established procedures that can be performed as analgesic or
anti-inflammatory procedures.
6.5.5.1. Time to Produce Effect: Immediate with local anesthesia, or within 3
days if no anesthesia.
6.5.5.2 Optimum Duration: Usually one to two injections is adequate.
6.5.5.3 Maximum Duration: Not more than three to four times annually.
6.5.5.4 Steroid injections should be used
cautiously in diabetic patients.
Diabetic patients should be reminded to check their blood-glucose level
at least twice daily for two weeks post-injections. Injections may be performed with or without
ultrasound guidance. Ultrasound guided interventional procedure provides the
ability to image soft tissues in real time and can improve safety and accuracy
of needle placement. The use of
ultrasound guided procedures will be at the discretion of the health care
provider.
6.5.6 Soft
Tissue Injections: include
bursa and tendon insertions. Injections under significant pressure should be
avoided as the needle may be penetrating the tendon. Injection into the tendon can cause tendon
degeneration, tendon breakdown, or rupture. Injections should be minimized for
patients younger than 30 years of age.
6.5.6.1 When performing tendon insertion
injections, the risk of tendon rupture should be discussed with the patient and
the need for restricted duty emphasized.
6.5.6.2 Time to Produce Effect: Immediate with
local anesthesia, or within 3 days if no anesthesia.
6.5.6.3 Optimum Duration: Usually
one to two injections is adequate.
6.5.6.4 Maximum Duration: Not more
than three to four times annually.
6.5.6.5 Steroid injections should be used
cautiously in diabetic patients.
Diabetic patients should be reminded to check their blood-glucose level
at least twice daily for two weeks post-injections. Injections may be performed with or without
ultrasound guidance. Ultrasound guided interventional procedure provides the
ability to image soft tissues in real time and can improve safety and accuracy
of needle placement. The use of
ultrasound guided procedures will be at the discretion of the health care
provider.
6.5.7 Trigger
Point Injections: although
generally accepted, have only rare indications in the treatment of lower
extremity disorders. Therefore, their
routine use is not recommended in the treatment of lower extremity injuries.
6.5.7.1 Description: Trigger point treatment can consist of dry
needling or injection of local anesthetic with or without corticosteroid into
highly localized, extremely sensitive bands of skeletal muscle fibers that
produce local and referred pain when activated.
Medication is injected in a four-quadrant manner in the area of maximum
tenderness. Injection efficacy can be
enhanced if injections are immediately followed by myofascial therapeutic
interventions, such as vapo-coolant spray and stretch, ischemic pressure
massage (myotherapy), specific soft tissue mobilization and physical
modalities. There is conflicting
evidence regarding the benefit of trigger point injections. A truly blinded study comparing dry needle
treatment of trigger points is not feasible.
There is no evidence that injection of medications improves the results
of trigger-point injections. Needling alone may account for some of the
therapeutic response.
6.5.7.2 There is no indication for conscious
sedation for patients receiving trigger point injections. The patient must be
alert to help identify the site of the injection.
6.5.7.3 Indications:
6.5.7.3.1 Trigger point injections may be used
to relieve myofascial pain and facilitate active therapy and stretching of the
affected areas. They are to be used as
an adjunctive treatment in combination with other treatment modalities such as
functional restoration programs. Trigger
point injections should be utilized primarily for the purpose of facilitating
functional progress. Patients should
continue with a therapeutic exercise program as tolerated throughout the time
period they are undergoing intensive myofascial interventions. Myofascial pain is often associated with
other underlying structural problems, and any abnormalities need to be ruled
out prior to injection.
6.5.7.3.2 Trigger point injections are
indicated in those patients where well circumscribed trigger points have been
consistently observed, demonstrating a local twitch response, characteristic
radiation of pain pattern and local autonomic reaction, such as persistent
hyperemia following palpation.
Generally, these injections are not necessary unless consistently
observed trigger points are not responding to specific, noninvasive, myofascial
interventions within approximately a 6 week time frame.
6.5.7.4 Complications: Potential but rare complications of trigger
point injections include infection, anaphylaxis, neurapraxia, and
neuropathy. If corticosteroids are
injected in addition to local anesthetic, there is a risk of developing local
myopathy. Severe pain on injection
suggests the possibility of an intraneural injection, and the needle should be
immediately repositioned.
6.5.7.5 Time
to Produce Effect: Local anesthetic 30
minutes; no anesthesia 24 to 48 hours.
6.5.7.6 Frequency: Weekly, suggest no more than 4 injection
sites per session per week to avoid significant post-injection soreness.
6.5.7.7 Optimum
Duration: 4 Weeks.
6.5.7.8 Maximum
Duration: 8 weeks. Occasional patients may require 2 to 4
repetitions of trigger point injection series over a 1 to 2 year period.
6.5.8 Viscosupplementation/Intracapsular Acid Salts: is an
accepted form of treatment for osteoarthritis or degenerative changes in the
knee joint. There is good evidence that
intra-articular hyaluronic acid injections have only a small effect on knee
pain and function. Therefore, the
patient and treating physician should identify functional goals and the
likelihood of achieving improved ability to perform activities of daily living
or work activities with injections versus other treatments. The patient should
agree to comply with the treatment plan including home exercise. These
injections may be considered an alternative in patients who have failed
non-operative treatment and surgery is not an option, particularly, if
non-steroidal anti-inflammatory drug treatment is contraindicated or has been
unsuccessful. Viscosupplementation is not recommended for patients with severe
osteoarthritis who are surgical candidates. Its efficacy beyond 6 months is not
well-established. There is no evidence
that one product significantly outperforms another, prior authorization is
required to approve product choice and for repeat series of injections.
6.5.8.1 One injection of 6 ml of Hylan G-F
20 may be effective and is an option for knee injections.
6.5.8.2 Viscosupplementation is not recommended
for ankle osteoarthritis due to the small effect size documented in knee
conditions and the lack of evidence supporting its use in the ankle.
Viscosupplementation is not recommended for hip arthritis given the probable
superiority of corticosteroid injections. In rare cases a patient with
significant hip osteoarthritis who does not qualify for surgical intervention
may try viscosupplementation. It should be done with ultrasound or
fluoroscopic guidance and will not necessarily require a series of three
injections. The patient may choose to have repeat injections when the first
injection was successful.
6.5.8.3 Time to Produce Effect: After 1 series or one injection as discussed
above, there must be a functional gain lasting three months to justify repeat
injections.
6.5.8.4 Frequency: One injection or 1 series (3 to 5 injections
generally spaced 1 week apart).
6.5.8.5 Optimum/Maximum
Duration: Varies. Efficacy beyond 6 months is not
well-established.
6.5.9 Prolotherapy: (also known as sclerotherapy) consists of peri-articular
injections of hypertonic dextrose with or without phenol with the goal of
inducing an inflammatory response that will recruit cytokine growth factors
involved in the proliferation of connective tissue. Advocates of prolotherapy propose that these
injections will alleviate complaints related to joint laxity by promoting the
growth of connective tissue and stabilizing the involved joint.
6.5.9.1 Laboratory studies may lend some
biological plausibility to claims of connective tissue growth, but high quality
published clinical studies are lacking.
The dependence of the therapeutic effect on the inflammatory response is
poorly defined, raising concerns about the use of conventional
anti-inflammatory drugs when proliferant injections are given. The evidence in support of prolotherapy is
insufficient and therefore, its use is not recommended in lower extremity
injuries.
6.6 JOBSITE ALTERATION
6.6.1 Early
evaluation and training of body mechanics are essential for every injured
worker. Risk factors to be addressed include: repetitive work, lifting, and
forces that have an impact on the lower extremity. In some cases, this requires a jobsite
evaluation. There is no single factor or combination of factors that is proven
to prevent or ameliorate lower extremity pain, but a combination of ergonomic and psychosocial factors are
generally considered to be important. Physical factors that may be considered
include use of force, repetitive work, squatting, climbing, kneeling,
crouching, crawling, prolonged standing, walking a distance or on uneven
surfaces, jumping, running, awkward positions requiring use of force, and lower
extremity vibration. Psychosocial
factors to be considered include pacing, degree of control over job duties,
perception of job stress, and supervisory support.
6.6.2 The
job analysis and modification should include input from the employee, employer,
and a medical professional familiar with work place evaluation. An ergonomist may also provide useful
information. The employee must be observed performing all job functions in
order for the jobsite analysis to be valid. Periodic follow-up is recommended
to evaluate effectiveness of the intervention and need for additional ergonomic
changes.
6.6.3 Interventions:
should consider engineering
controls (e.g., mechanizing the task, changing the tool used, or adjusting the
jobsite), or administrative controls (e.g., adjusting the time an individual
performs the task).
6.7 MEDICATIONS AND MEDICAL MANAGEMENT
6.7.1 Use
of medications will vary widely due to the spectrum of injuries from simple
strains to complicated fractures. A
thorough medication history, including use of alternative and over-the-counter
medications, should be performed at the time of the initial visit and updated
periodically. Treatment for pain control is initially accomplished with
acetaminophen and/or NSAIDs. The patient should be educated regarding the
interaction with prescription and over-the-counter medications as well as the
contents of over-the-counter herbal products.
6.7.2 Nonsteroidal anti-inflammatory
drugs (NSAIDs) and acetaminophen are useful in the treatment of injuries
associated with degenerative joint disease and/or inflammation. These same medications can be used for pain
control.
6.7.3 Topical agents can be
beneficial for pain management in lower extremity injuries. This includes topical capsaicin,
nonsteroidals, as well as topical iontphoretics/phonophoretics, such as steroid
creams and lidocaine.
6.7.4 Glucosamine and chondroitin are
sold in the United States
as dietary supplements. Their dosage, manufacture, and purity are not regulated
by the Food and Drug Administration. For moderate to severe knee
osteoarthritis, there is good evidence for the effectiveness of a
pharmaceutical grade combination of 500 mg glucosamine hydrochloride and 400 mg
chondroitin sulfate three times per day. Effectiveness for mild disease is
unknown. Recent literature suggests that chondroitin sulfate in a dose of 800
mg once daily may reduce the rate of joint degradation as demonstrated by joint
space loss on serial x-rays.
6.7.5 For mild-to-moderate
osteoarthritis confined to the hip, there is good evidence that a
pharmaceutical-grade glucosamine sulfate is unlikely to produce a clinically
significant improvement in pain and joint function.
6.7.6 When osteoarthritis is
identified as a contributing factor to a work–related injury, pharmaceutical
grade glucosamine and chondroitin may be tried.
Long-term coverage for these medications would fall under Workers’
Compensation only when the arthritic condition is primarily related to the work
injury.
6.7.7 S-adenosyl methionine (SAM-e),
like glucosamine and chondroitin, is sold as a dietary supplement in the United States,
with a similar lack of standard preparations of dose and manufacture. There is
some evidence that a pharmaceutical-grade SAM-e is as effective as celecoxib in
improving pain and function in knee osteoarthritis, but its onset of action is
slower. Studies using liquid chromatography have shown that it may lose its
potency after several weeks of storage. In addition, SAM-e has multiple
additional systemic effects. It is not
currently recommended due to lack of availability of pharmaceutical quality,
systemic effects, and loss of potency with storage.
6.7.8 The following medications and medical
management items are listed in alphabetical order.
6.7.8.1 Acetaminophen is an effective analgesic with antipyretic but not
anti-inflammatory activity.
Acetaminophen is generally well tolerated, causes little or no
gastrointestinal irritation and is not associated with ulcer formation. Acetaminophen has been associated with liver
toxicity in overdose situations or in chronic alcohol use. Patients may not
realize that many over-the-counter preparations may contain acetaminophen. The
total daily dose of acetaminophen is recommended not to exceed 4 grams per
24-hour period, from all sources, including narcotic-acetaminophen combination
preparations.
6.7.8.1.1 Optimal Duration: 7 to 10 days.
6.7.8.1.2 Maximum Duration: Chronic use as
indicated on a case-by-case basis. Use of this substance long-term for 3 days
per week or greater may be associated with rebound pain upon cessation.
6.7.8.2. Bisphosphonates may be used for
those qualifying under osteoporosis guidelines.
Long-term use for the purpose of increasing prosthetic fixation is not
recommended as long-term improvement in fixation is not expected. See Section 7, h., Osteoporosis Management
Section below.
6.7.8.3 Deep Venous Thrombosis Prophylaxis is a complex issue involving many variables
such as individual patient characteristics, the type of surgery, anesthesia
used and agent(s) used for prophylaxis.
Final decisions regarding prophylaxis will depend on the surgeon’s
clinical judgment. The following are
provided as generally accepted concepts regarding prophylaxis at the time of
writing of these guidelines.
6.7.8.3.1 All patients undergoing lower
extremity surgery or prolonged lower extremity immobilization should be
evaluated for elevated risk for DVT and should receive education on prevention.
Possible symptoms should be discussed.
Patients at higher risk than the normal population include, but are not
limited to, those with known hypercoagulable states and those with previous
pulmonary embolism or DVT. Those considered at higher risk for bleeding, which
may alter thromboprophylaxis protocols, include patients with a history of a
bleeding disorder, recent gastrointestinal bleed, or hemorrhagic stroke.
6.7.8.3.2 There is no evidence to support
mandatory prophylaxis for all patients who are immobilized or undergo lower
extremity procedures, outside of hip or knee arthroplasties or hip fracture
repair.
6.7.8.3.3 Hip and knee arthroplasties and hip
fracture repair are standard risk factors requiring thromboprophylaxis.
Commonly used agents are low molecular weight heparin, low dose un-fractionated
heparin (LDUH), synthetic pentasaccaride fondaparinux, or warfarin. If aspirin
is used, it should be accompanied by aggressive mechanical prophylaxis.
6.7.8.3.4 All patients should be mobilized as
soon as possible after surgery.
Mechanical prophylaxis such as pneumatic devices that are thigh calf,
calf only, or foot pumps may be considered immediately post-operatively and/or
until the patient is discharged home.
Thigh length or knee high graduated compression stockings are used for
most patients. With prolonged
prophylaxis, lab tests must be drawn regularly.
These may be accomplished with home health care or outpatient
laboratories when appropriate.
6.7.8.4 Minor Tranquilizer/Muscle Relaxants are appropriate for muscle
spasm, mild pain and sleep disorders. When prescribing these agents, physicians
must seriously consider side effects of drowsiness or dizziness and the fact
that benzodiazepines may be habit-forming.
6.7.8.4.1 Optimal Duration: 1 week.
6.7.8.4.2 Maximum Duration: 4 weeks.
6.7.8.5 Narcotics
should be primarily reserved for the treatment of severe lower extremity
pain. There are circumstances where
prolonged use of narcotics is justified based upon specific diagnosis, and in
these cases, it should be documented and justified. In mild-to-moderate cases of lower extremity
pain, narcotic medication should be used cautiously on a case-by-case
basis. Adverse effects include
respiratory depression, the development of physical and psychological
dependence, and impaired alertness.
6.7.8.5.1 Narcotic medications should be
prescribed with strict time, quantity, and duration guidelines, and with
definitive cessation parameters. Pain is subjective in nature and should be
evaluated using a pain scale and assessment of function to rate effectiveness
of the narcotic prescribed. The physician should refer to the chronic pain
guidelines, when appropriate.
6.7.8.5.2 Optimal Duration: 1 week
6.7.8.5.3 Maximum Duration: 4 weeks.
6.7.8.5.4 Use beyond 4 weeks is acceptable in
appropriate cases. Chronic use may be
appropriate in selected individual cases.
6.7.8.6 Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) are useful for pain and
inflammation. In mild cases, they may be
the only drugs required for analgesia.
There are several classes of NSAIDs, and the response of the individual
injured worker to a specific medication is unpredictable. For this reason, a range of NSAIDs may be
tried in each case with the most effective preparation being continued. Patients should be closely monitored for
adverse reactions. The US Food and Drug
Administration advise that many NSAIDs may cause an increased risk of serious
cardiovascular thrombotic events, myocardial infarction, and stroke, which can
be fatal. Administration of proton pump
inhibitors, histamine 2 blockers, or prostaglandin analog misoprostol along
with these NSAIDs may reduce the risk of duodenal and gastric ulceration but do
not impact possible cardiovascular complications. Due to the cross-reactivity between aspirin and
NSAIDs, NSAIDs should not be used in aspirin-sensitive patients, and should be
used with caution in all asthma patients.
NSAIDs are associated with abnormal renal function, including renal
failure, as well as, abnormal liver function.
Certain NSAIDs may have interactions with various other
medications. Individuals may have
adverse events not listed above. Intervals for metabolic screening
are dependent upon the patient's age, general health status and should be
within parameters listed for each specific medication. Complete Blood Count (CBC) and liver and renal function should be
monitored at least every six months in patients on chronic NSAIDs and initially
when indicated.
6.7.8.6.1 NSAIDs may be used for pain
management after joint replacement. They have also been used to reduce
heterotopic ossification after arthroplasty. NSAIDs do reduce the
radiographically documented heterotopic ossification in this setting, but there
is some evidence that they do not improve functional outcomes and they may increase
the risk of bleeding events in the post-operative period. Their routine use for prevention of
heterotopic bone formation is not recommended.
6.7.8.6.2 Non-selective
Nonsteroidal Anti-Inflammatory Drugs:
6.7.8.6.2.1 Includes NSAIDs and acetylsalicylic
acid (aspirin). Serious GI toxicity,
such as bleeding, perforation, and ulceration can occur at any time, with or
without warning symptoms in patients treated with traditional NSAIDs. Physicians should inform patients about the
signs and/or symptoms of serious gastrointestinal toxicity and what steps to
take if they occur. Anaphylactoid
reactions may occur in patients taking NSAIDs.
NSAIDs may interfere with platelet function. Fluid retention and edema have been observed
in some patients taking NSAIDs.
6.7.8.6.2.2 Optimal Duration: 1 week.
6.7.8.6.2.3 Maximum Duration: 1 year.
Chronic use is appropriate in individual cases. Use of these substances long-term (3 days per
week or greater) is associated with rebound pain upon cessation.
6.7.8.6.3 Selective
Cyclo-oxygenase-2 (COX-2) Inhibitors:
6.7.8.6.3.1 COX-2 inhibitors are more recent
NSAIDs and differ in adverse side effect profiles from the traditional
NSAIDs. The major advantages of
selective COX-2 inhibitors over traditional NSAIDs are that they have less
gastrointestinal toxicity and no platelet effects. COX-2 inhibitors can worsen renal function in
patients with renal insufficiency, thus renal function may need monitoring.
6.7.8.6.3.2 COX-2 inhibitors should not be
first-line for low risk patients who will be using a NSAID short-term but are
indicated in select patients for whom
traditional NSAIDs are not tolerated.
Serious upper GI adverse events can occur even in asymptomatic
patients. Patients at high risk for GI
bleed include those who use alcohol, smoke, are older than 65, take
corticosteroids or anti-coagulants, or have a longer duration of therapy. Celecoxib
is contraindicated in sulfonamide allergic patients.
6.7.8.6.3.3 Optimal Duration: 7 to 10 days.
6.7.8.6.3.4 Maximum Duration: Chronic use is appropriate in individual
cases. Use of these substances long-term
(3 days per week or greater) is associated with rebound pain upon cessation.
6.7.8.7 Oral Steroids have limited use but are accepted in cases
requiring potent anti-inflammatory drug effect in carefully selected
patients. A one-week regime of steroids
may be considered in the treatment of patients who have arthritic flare-ups
with significant inflammation of the joint.
The physician must be fully aware of potential contraindications for the
use of all steroids such as hypertension, diabetes, glaucoma, peptic ulcer
disease, etc., which should be discussed with the patient.
6.7.8.7.1 Optimal Duration: 3 to 7 days.
6.7.8.7.2 Maximum Duration: 7 days.
6.7.8.8 Osteoporosis Management:
6.7.8.8.1 All patients with conditions which
require bone healing, especially those over 50, should be encouraged to ingest
at least 1200 mg of Calcium and 800 IU of Vitamin D per day. There is some evidence that, for women in the
older age group (58 to 88) with low hip bone density, greater callus forms for
those who adhere to these recommendations than those who do not. Although the clinical implications of this
are not known, there is greater non-union in this age group and thus, coverage
for these medications during the fracture healing time period is
recommended. At this time there is no
evidence that bisphosphonates increase acute fracture healing.
6.7.8.8.2 Female patients over 65 should be
referred for an osteoporosis evaluation if one has not been completed the
previous year. Patients who have been on
prednisone at a dose of 5 to 7.5 mg for more than 3 months should be evaluated
for glucocorticoid induced osteoporosis.
An osteoporosis evaluation may be considered for males who: are over 70,
are physically inactive, have previous fragility fracture, have a BMI less than
20, or have been hypogonadal for 5 years. Evaluation may also be considered for
patients on medications that can cause bone loss, patients who have suffered a
fracture due to a low-impact fall or with minimum to no provocation, and women
under 65 with one of the following: menopause before 40, current smoker, or
body mass index less than 20. Low body weight appears to be the best predictor
of osteoporosis in women younger that 65. In one adequate study, all patients
aged 50 to 75 referred to an orthopaedic department for treatment of wrist,
vertebral, proximal humerus, or hip fractures received bone mass density
testing. 97% of patients had either osteoporosis
(45%) or osteopenia (42%). Referral is important to prevent future factures in
these groups. Long-term care for osteoporosis is not covered under workers
compensation even though it may be discovered due to an injury-related acute
fracture.
6.7.8.9 Psychotropic/Anti-anxiety/Hypnotic Agents may be useful for
treatment of mild and chronic pain, dysesthesias, sleep disorders, and
depression. Post-operative
patients may receive medication to assure normal sleep cycles. Antidepressant
medications, such as tricyclics and Selective Serotonin Reuptake Inhibitors
(SSRIs), are useful for affective disorder and chronic pain management. Tricyclic antidepressant agents, in low dose,
are useful for chronic pain but have more frequent side effects.
6.7.8.9.1 Anti-anxiety medications are best
used for short-term treatment (i.e., less than 6 months). Accompanying sleep disorders are best treated
with sedating antidepressants prior to bedtime.
Frequently, combinations of the above agents are useful. As a general rule, physicians should access
the patient’s prior history of substance abuse or depression prior to
prescribing any of these agents.
6.7.8.9.2 Due to the habit-forming potential of
the benzodiazepines and other drugs found in this class, they are not routinely
recommended. Refer to the Chronic Pain Guidelines which give a detailed
discussion regarding medication use in chronic pain management.
6.7.8.9.3 Optimal Duration: 1 to 6 months.
6.7.8.9.4 Maximum Duration: 6 to 12 months, with monitoring.
6.7.8.10 Topical Drug Delivery: Creams and patches may be an alternative
treatment of localized musculoskeletal disorders. It is necessary that all topical agents be
used with strict instructions for application as well as the maximum number of
applications per day to obtain the desired benefit and avoid potential
toxicity. As with all medications,
patient selection must be rigorous to select those patients with the highest
probability of compliance. Refer to
“Iontophoresis” in “Passive Therapy” part of this section for information
regarding topical iontophoretic agents.
6.7.8.10.1 Topical
Salicylates and Nonsalicylates have been shown to be effective in relieving
pain in acute and chronic musculoskeletal conditions. Topical salicylate and nonsalicylates achieve
tissue levels that are potentially therapeutic, at least with regard to COX
inhibition. Other than local skin
reactions, the side effects of therapy are minimal, although not non-existent,
and the usual contraindications to use of these compounds needs to be
considered. Local skin reactions are
rare and systemic effects were even less common. Their use in patients receiving warfarin
therapy may result in alterations in bleeding time. Overall, the low level of systemic absorption
can be advantageous; allowing the topical use of these medications when
systemic administration is relatively contraindicated such as is the case in
patients with hypertension, cardiac failure, or renal insufficiency.
6.7.8.10.1.1 There
is no evidence that topical agents are more or less effective than oral
medications.
6.7.8.10.1.2 Optimal Duration: 1 week.
6.7.8.10.1.3 Maximal Duration: 2 weeks per episode.
6.7.8.10.2 Capsaicin
is another medication option for topical drug use in lower extremity
injury. Capsaicin offers a safe and effective alternative to systemic NSAID
therapy. Although it is quite safe, effective use of capsaicin is limited by
the local stinging or burning sensation that typically dissipates with regular
use, usually after the first 7 to 10 days of treatment. Patients should be advised to apply the cream
on the affected area with a plastic glove or cotton applicator and to avoid
inadvertent contact with eyes and mucous membranes.
6.7.8.10.2.1 Optimal Duration: 1 week.
6.7.8.10.2.2 Maximal Duration: 2 weeks per episode.
6.7.8.10.3 Iontophoretic
Agents: Refer to “Iontophoresis,” in
this Section 6.0, under the subsection for Passive Therapy.
6.7.8.11 Tramadol is useful in relief of lower
extremity pain and has been shown to provide pain relief equivalent to
that of commonly prescribed NSAIDs. Tramadol
is an atypical opioid with norepinephrine and serotonin reuptake inhibition. It
is not considered a controlled substance in the U.S.
Although Tramadol may cause impaired alertness, it is
generally well tolerated, does not cause gastrointestinal ulceration, or
exacerbate hypertension or congestive heart failure. Tramadol should be used cautiously in
patients who have a history of seizures or who are taking medication that may
lower the seizure threshold, such as MAO inhibitors, SSRIs, and tricyclic
antidepressants. This medication has
physically addictive properties and withdrawal may follow abrupt
discontinuation and is not recommended for patients with prior opioid
addiction.
6.7.8.11.1 Optimal Duration: 3 to 7
days.
6.7.8.11.2 Maximum Duration: 2 weeks.
Use beyond 2 weeks is acceptable in appropriate cases.
6.8 OCCUPATIONAL
REHABILITATION PROGRAMS
6.8.1 Non-Interdisciplinary:
These generally accepted programs
are work-related, outcome-focused, individualized treatment programs. Objectives of the program include, but are
not limited to, improvement of cardiopulmonary and neuromusculoskeletal
functions (strength, endurance, movement, flexibility, stability, and motor
control functions), patient education, and symptom relief. The goal is for patients to gain full or
optimal function and return to work. The
service may include the time-limited use of passive modalities with progression
to achieve treatment and/or simulated/real work.
6.8.1.1 Work Conditioning: These
programs are usually initiated once reconditioning has been completed but may
be offered at any time throughout the recovery phase. It should be initiated when imminent return
of a patient to modified- or full-duty is not an option, but the prognosis for
returning the patient to work at completion of the program is at least fair to
good.
6.8.1.1.1 Length of visit: 1 to 2 hours per day.
6.8.1.1.2 Frequency: 2 to 5 visits per week.
6.8.1.1.3 Optimum Duration: 2 to 4 weeks.
6.8.1.1.4 Maximum Duration: 6 weeks.
Participation in a program beyond six weeks must be documented with
respect to need and the ability to facilitate positive symptomatic or
functional gains.
6.8.1.2 Work Simulation: is a
program where an individual completes specific work-related tasks for a
particular job and return-to-work. Use
of this program is appropriate when modified duty can only be partially
accommodated in the work place, when modified duty in the work place is
unavailable, or when the patient requires more structured supervision. The need for work place simulation should be
based upon the results of a Functional Capacity Evaluation and/or Jobsite
Analysis.
6.8.1.2.1 Length of visit: 2 to 6 hours per day.
6.8.1.2.2 Frequency: 2 to 5 visits per week.
6.8.1.2.3 Optimum Duration: 2 to 4 weeks.
6.8.1.2.4 Maximum Duration: 6 weeks.
Participation in a program beyond six weeks must be documented with
respect to need and the ability to facilitate positive symptomatic or functional
gains.
6.9 ORTHOTICS AND PROSTHETICS
6.9.1 Fabrication/Modification of Orthotics:
would be used when there is need to normalize weight-bearing, facilitate
better motion response, stabilize a joint with insufficient muscle or
proprioceptive/reflex competencies, to protect subacute conditions as needed
during movement, and correct biomechanical problems. Footwear modifications may be necessary for
work shoes and everyday shoes.
Replacement is needed every six months to one year. For specific types
of orthotics/prosthetics see Section 5.0,
Specific Lower Extremity Injury Diagnosis, Testing and Treatment.
6.9.1.1 Time to Produce Effect: 1 to
3 sessions (includes wearing schedule and evaluation).
6.9.1.2 Frequency: 1 to 2 times per
week.
6.9.1.3 Optimum/Maximum Duration: Over a period of approximately 4 to 6 weeks
for casting, fitting, and re-evaluation.
6.9.2 Orthotic/Prosthetic
Training: is the skilled instruction (by qualified
providers) in the proper use of orthotic devices and/or prosthetic limbs
including stump preparation, donning and doffing limbs, instruction in wearing
schedule and orthotic/prosthetic maintenance training. Training can include gait, mobility, transfer
and self-care techniques.
6.9.2.1 Time to Produce Effect: 2 to 6 sessions.
6.9.2.2 Frequency: 3 times per week.
6.9.2.3 Optimum/Maximum
Duration: 2 to 4 months.
6.9.3 Splints or Adaptive Equipment design,
fabrication and/or modification indications include
the need to control neurological
and orthopedic injuries for reduced stress during functional activities and
modify tasks through instruction in the use of a device or physical
modification of a device, which reduces stress on the injury. Equipment should improve safety and reduce
risk of re-injury. This includes high
and low technology assistive options such as workplace modifications, crutch or
walker training, and self-care aids.
6.9.3.1 Time to Produce Effect: Immediate.
6.9.3.2 Frequency: 1 to 3 sessions or as indicated to establish
independent use.
6.9.3.3 Optimum/Maximum
Duration: 1 to 3 sessions.
6.10 PATIENT
EDUCATION: No treatment
plan is complete without addressing issues of individual and/or group patient
education as a means of prolonging the beneficial effects of treatment, as well
as facilitating self-management of symptoms and injury prevention. The patient should be encouraged to take an
active role in the establishment of functional outcome goals. They should be educated on their specific
injury, assessment findings, and plan of treatment. Instruction on proper body mechanics and
posture, positions to avoid, self-care for exacerbation of symptoms, and home
exercise should also be addressed.
6.10.1 Time to Produce Effect: Varies with individual patient.
6.10.2 Frequency: Should occur at each visit.
6.11 PERSONALITY-PSYCHOSOCIAL-PSYCHOLOGICAL
INTERVENTION: Psychosocial
treatment is a generally accepted, widely used and well-established
intervention. This group of therapeutic
and diagnostic modalities includes, but is not limited to: individual
counseling, group therapy, stress management, psychosocial crises intervention,
hypnosis and meditation. Any screening
or diagnostic workup should clarify and distinguish between pre-existing versus
aggravated versus purely causative psychological conditions. Psychosocial intervention is recommended as
an important component in the total management program that should be
implemented as soon as the problem is identified. This can be used alone or in
conjunction with other treatment modalities.
Providers treating patients with chronic pain should refer to the Delaware
Workers’ Compensation practice guidelines for Chronic Pain. Time to Produce
Effect: 2 to 4 weeks.
6.11.1 Frequency: 1 to 3 times weekly for the first 4 weeks
(excluding hospitalization, if required), decreasing to 1 to 2 times per week
for the second month. Thereafter, 2 to 4
times monthly.
6.11.2 Optimum Duration: 6 weeks to 3 months.
6.11.3 Maximum Duration: 3 to 12 months. Counseling is not intended to delay but to
enhance functional recovery. For select
patients, longer supervised treatment may be required, and if further
counseling beyond 3 months is indicated, documentation addressing which
pertinent issues are pre-existing versus aggravated versus causative, as well
as projecting a realistic functional prognosis, should be provided by the
authorized treating provider every 4 to 6 weeks during treatment.
6.12 RESTRICTION OF ACTIVITIES
varies according to the specific diagnosis and the severity of the
condition. Job modification/modified
duty are frequently required to avoid exacerbation of the injured lower
extremity. Complete work cessation should be avoided, if possible, since it
often further aggravates the pain presentation.
Modified return-to-work is almost always more efficacious and rarely
contraindicated in the vast majority of injured workers with lower extremity
injuries.
6.13 RETURN-TO-WORK: Early return-to-work should be a
prime goal in treating occupational injuries given the poor return-to-work
prognosis for an injured worker who has been out of work for more than six
months. It is imperative that the
patient be educated regarding the benefits of return-to-work, work
restrictions, and follow-up if problems arise.
When attempting to return a patient to work after a specific injury,
clear objective restrictions of activity level should be made. An accurate job description with detailed
physical duty restrictions is often necessary to assist the
physician in making return-to-work recommendations. This may require a jobsite evaluation.
6.13.1 Employers
should be prepared to offer transitional work. This may consist of temporary
work in a less demanding position, return to the regular job with restrictions,
or gradual return to the regular job. Company policies which encourage
return-to-work with positive communication are most likely to have decreased
worker disability.
6.13.2 Return-to-work is defined as any
work or duty that the patient is able to perform safely. It may not be the patient’s regular work. Due to the large spectrum of injuries of
varying severity and varying physical demands in the work place, it is not
possible to make specific return-to-work guidelines for each injury.
6.14 THERAPY
– ACTIVE: The
following active therapies are widely used and accepted methods of care for a
variety of work-related injuries. They
are based on the philosophy that therapeutic exercise and/or activity are
beneficial for restoring flexibility, strength, endurance, function,
range-of-motion, and can alleviate discomfort.
Active therapy requires an internal effort by the individual to complete
a specific exercise or task. This form
of therapy requires supervision from a therapist or medical provider such as
verbal, visual and/or tactile instruction(s).
At times, the provider may help stabilize the patient or guide the
movement pattern but the energy required to complete the task is predominately
executed by the patient.
Patients should
be instructed to continue active therapies at home as an extension of the treatment
process in order to maintain improvement levels. Follow-up visits to reinforce and monitor
progress and proper technique are recommended. Home exercise can
include exercise with or without mechanical assistance or resistance and
functional activities with assistive devices.
The following
active therapies are listed in alphabetical order:
6.14.1 Activities of Daily Living (ADL)
are well-established interventions which involve instruction,
active-assisted training, and/or adaptation of activities or equipment to
improve a person's capacity in normal daily activities such as self-care, work
re-integration training, homemaking, and
driving.
6.14.1.1 Time to Produce Effect: 4 to 5 treatments.
6.14.1.2 Maximum Duration: 6 weeks.
6.14.2 Aquatic Therapy: is a well-accepted treatment which consists
of the therapeutic use of aquatic immersion for therapeutic exercise to promote
ROM, flexibility, core stabilization, endurance, strengthening, body mechanics,
and pain management. Aquatic therapy
includes the implementation of active therapeutic procedures in a swimming or
therapeutic pool. The water provides a
buoyancy force that lessens the amount of force gravity applies to the body. The decreased gravity effect allows the
patient to have a mechanical advantage and more likely to have a successful
trial of therapeutic exercise. Studies
have shown that the muscle recruitment for aquatic therapy versus similar
non–aquatic motions is significantly less.
Because there is always a risk of recurrent or additional damage to the
muscle tendon unit after a surgical repair, aquatic therapy may be preferred by
physicians to gain early return of ROM. In some
cases the patient will be able to do the exercises unsupervised after the
initial supervised session. Parks and recreation contacts may be used to locate
less expensive facilities for patients.
6.14.2.1 Indications include the
following:
6.14.2.1.1 Post-operative
therapy as ordered by the physician; or
6.14.2.1.2 Intolerance
for active land-based or full-weight-bearing therapeutic procedures; or
6.14.2.1.3 Symptoms that are
exacerbated in a dry environment; and
6.14.2.1.4 Willingness to follow
through with the therapy on a regular basis.
6.14.2.2 The
pool should be large enough to allow full extremity ROM and fully erect
posture. Aquatic vests, belts, snorkels,
and other devices may be used to provide stability, balance, buoyancy, and
resistance.
6.14.2.3 Time
to Produce Effect: 4 to 5 treatments.
6.14.2.4 Frequency: 3 to 5 times per week.
6.14.2.5 Maximum
Duration: 25 Visits
6.14.2.6 A
physician prescribed self-directed program is recommended after the supervised
aquatics program has been established, or alternatively a transition to a
self-directed dry environment exercise program.
6.14.2.7 There is some evidence that for
osteoarthritis of the hip or knee, aquatic exercise probably slightly reduces
pain and slightly improves function over 3 months.
6.14.3 Functional
Activities are the use of therapeutic activity to enhance mobility,
body mechanics, employability, coordination, balance, and sensory motor
integration.
6.14.3.1 Time to Produce Effect: 4 to 5 treatments
6.14.3.2 Frequency: 3 to 5 times per week.
6.14.3.3 Maximum Duration: 24 visits
6.14.3.4 Total
number of visit 97110 and 97530 should not exceed 40 visits without preauthorization.
6.14.4 Functional Electrical Stimulation
is the application of electrical current to elicit involuntary or assisted
contractions of atrophied and/or impaired muscles. Indications include muscle atrophy, weakness,
sluggish muscle contraction, neuromuscular dysfunction or peripheral nerve
lesion. Indications also may include an
individual who is precluded from active therapy.
6.14.4.1 Time to Produce Effect: 2 to 6 treatments.
6.14.4.2 Frequency: 3 times per week.
6.14.4.3 Maximum
Duration: 24 visits inclusive of
electrical muscle stimulation codes.. If
beneficial, provide with home unit.
6.14.5 Gait Training is specialized training that promotes normal gait
for a person with a faulty gait pattern secondary to lower extremity injury or
surgery. Indications include the need to
promote normal gait pattern with or without assistive devices; instruct in the
safety and proper use of assistive devices; instruct in progressive use of more
independent devices (i.e., platform-walker, to walker, to crutches, to cane);
instruct in gait on uneven surfaces and steps (with and without railings) to
reduce risk of fall, or loss of balance; and/or instruct in equipment to limit
weight-bearing for the protection of a healing injury or surgery.
6.14.5.1 Time to Produce Effect: 2 to 6 treatments.
6.14.5.2 Frequency: 2 to 3 times per week.
6.14.5.3 Maximum Duration: 12 Visits
6.14.6 Neuromuscular Re-education is the skilled
application of exercise with manual, mechanical, or electrical facilitation to
enhance strength; movement patterns; neuromuscular response;
proprioception; kinesthetic sense; coordination; education of movement, balance and
posture. Indications include the need to
promote neuromuscular responses through carefully timed proprioceptive stimuli
to elicit and improve motor activity in patterns similar to normal
neurologically developed sequences, and improve neuromotor response with
independent control.
6.14.6.1 Time to Produce Effect: 2 to 6 treatments.
6.14.6.2 Frequency: 3-5 times per week.
6.14.6.3 Maximum Duration: 36 visits.
6.14.7 Therapeutic Exercise is a
generally accepted treatment with or without mechanical
assistance or resistance, may include isoinertial, isotonic, isometric and
isokinetic types of exercises. There
is good evidence to support the functional benefits of manual therapy with
exercise, walking programs, conditioning, and other combined therapy
programs. Indications include the need
for cardiovascular fitness, reduced edema, improved muscle strength, improved
connective tissue strength and integrity, increased bone density, promotion of
circulation to enhance soft tissue healing, improvement of muscle recruitment,
increased range of motion and are used to promote normal movement patterns. May
also include complementary/alternative exercise movement therapy.
6.14.7.1 Time to Produce Effect: 2 to 6 treatments.
6.14.7.2 Frequency: 3 to 5 times per week.
6.14.7.3 Maximum Duration: 36 visits.
6.14.7.4 Total number of visits of
97110 & 97530 may not exceed 40 visits without pre-authorization
6.14.8 Wheelchair Management and Propulsion is the instruction and training of
self-propulsion and proper use of a wheelchair.
This includes transferring and safety instruction. This is indicated in individuals who are not
able to ambulate due to bilateral lower extremity injuries, inability to use
ambulatory assistive devices, and in cases of multiple traumas.
6.14.8.1 Time to Produce Effect: 2 to 6 treatments.
6.14.8.2 Frequency: 2 to 3 times per week..
6.14.8.3 Maximum Duration: 6 visits.
6.15 THERAPY – PASSIVE: Most of the following passive therapies and
modalities are generally well-accepted methods of care for a variety of
work-related injuries. Passive therapy
includes those treatment modalities that do not require energy expenditure on
the part of the patient. They are
principally effective during the early phases of treatment and are directed at
controlling symptoms such as pain, inflammation and swelling and to improve the
rate of healing soft tissue injuries.
They should be use adjunctively with active therapies to help control
swelling, pain, and inflammation
during the rehabilitation process. They
may be used intermittently as the provider deems appropriate or regularly if
there are specific goals with objectively measured functional improvements
during treatment.
On occasion,
specific diagnoses and post-surgical conditions may warrant durations of
treatment beyond those listed as "maximum.” Factors such as exacerbation of symptoms,
re-injury, interrupted continuity of care and comorbidities may also extend
durations of care. Specific goals with
objectively measured functional improvement during treatment must be cited to
justify extended durations of care. It
is recommended that, if no functional gain is observed after the number of
treatments under “time to produce effect” has been completed alternative
treatment interventions, further diagnostic studies, or further consultations
should be pursued.
The following
passive therapies and modalities are listed in alphabetical order.
6.15.1 Continuous Passive Motion (CPM) is a form of passive
motion using specialized machinery that acts to move a joint and may also pump
blood and edema fluid away from the joint and periarticular tissues. CPM is effective in preventing the
development of joint stiffness if applied immediately following surgery. It should be continued until the swelling
that limits motion of the joint no longer develops. ROM for the joint begins at the level of
patient tolerance and is increased twice a day as tolerated. Home use of CPM is expected after chondral
defect surgery. CPM may be necessary for
cases with ACL repair, manipulation, joint replacement or other knee surgery if
the patient has been non compliant with pre-operative ROM exercises. Use of this equipment may require home
visits.
6.15.1.1 Time to Produce Effect: Immediate.
6.15.1.2 Frequency: Up to 4 times a day.
6.15.1.3 Optimum Duration: Up to 3 weeks post surgical.
6.15.1.4 Maximum Duration: 3 weeks.
6.15.2 Contrast Baths can be used for alternating
immersion of extremities in hot and cold water.
Indications include edema in the sub-acute stage of healing, the need to
improve peripheral circulation and decrease joint pain and stiffness.
6.15.2.1 Time to Produce Effect: 3 treatments.
6.15.2.2 Frequency: 3 times per week.
6.15.2.3 Maximum Duration: 24 visits
6.15.3 Electrical Stimulation
(Attended and Unattended), an
accepted treatment; once applied, requires minimal on-site supervision
by the physician or non-physician provider.
Indications include pain, inflammation, muscle spasm, atrophy, decreased
circulation, and the need for osteogenic stimulation.
6.15.3.1 Time to Produce Effect: 2 to 4 treatments.
6.15.3.2 Maximum Duration: 24 visits
6.15.4 Fluidotherapy employs a stream of dry,
heated air that passes over the injured body part. The injured body part can be exercised during
the application of dry heat. Indications
include the need to enhance collagen extensibility before stretching, reduce
muscle guarding, or reduce inflammatory response.
6.15.4.1 Time to Produce Effect: 1 to 4 treatments.
6.15.4.2 Frequency 1-5 times per week
6.15.4.3 Maximum Duration: 24 visits.
6.15.5 Iontophoresis is the transfer of
medication, including, but not limited to, steroidal anti-inflammatory and
anesthetics, through the use of electrical stimulation. Indications include pain (Lidocaine),
inflammation (hydrocortisone, salicylate), edema (mecholyl, hyaluronidase, and
salicylate), ischemia (magnesium, mecholyl, and iodine), muscle spasm
(magnesium, calcium); calcific deposits (acetate), scars, and keloids (chlorine, iodine, acetate).
6.15.5.1 Time to Produce Effect: 1 to 4 treatments.
6.15.5.2 Frequency: 3 times per week with at least 48 hours
between treatments.
6.15.5.3 Maximum Duration: 8 visits per body region
6.15.6. LASER: There is mounting evidence that low-level
LASER treatment may be useful in tendonopathy, trigger points, nerve injury,
and osteoarthritis of the knee.
Low-level LASER treatment is therefore an accepted treatment for the
above.
6.15.6.1 Time
to produce effect: 4-8 treatments
6.15.6.2 Frequency: 3 sessions per week
6.15.6.3 Maximum
Duration: 18 visits.
6.15.7 Manual Therapy Techniques
are passive interventions in which the providers use his or her hands, with or
without instruments, to administer skilled movements designed to modulate pain;
increase joint range of motion; reduce/eliminate soft tissue swelling, inflammation,
or restriction; induce relaxation; and improve contractile and non-contractile
tissue extensibility. These techniques are applied only after a thorough
examination is performed to identify those for whom manual therapy would be
contraindicated or for whom manual therapy must be applied with caution.
6.15.7.1 Manipulation is generally accepted,
well-established and widely used therapeutic intervention for lower extremity
pain and dysfunction. Manipulative Treatment (not therapy) is defined as the
therapeutic application of manually guided forces by an operator to improve
physiologic function and/or support homeostasis that has been altered by the
injury or occupational disease, and has associated clinical significance.
6.15.7.1.1 High
velocity, low amplitude (HVLA) technique, chiropractic manipulation,
osteopathic manipulation, muscle energy techniques, counter strain, and
non-force techniques are all types of manipulative treatment. This may be applied by osteopathic physicians
(D.O.), chiropractic physicians (D.C.), properly trained physical therapists
(P.T.), or properly trained medical physicians.
Under these different types of manipulation exist many subsets of
different techniques that can be described as a) direct- a forceful engagement
of a restrictive/pathologic barrier, b) indirect- a gentle/non-forceful
disengagement of a restrictive/pathologic barrier, c) the patient actively
assists in the treatment and d) the patient relaxing, allowing the practitioner
to move the body tissues. When the
proper diagnosis is made and coupled with the appropriate technique,
manipulation has no contraindications and can be applied to all tissues of the
body. Pre-treatment assessment should be
performed as part of each manipulative treatment visit to ensure that the
correct diagnosis and correct treatment is employed.
6.15.7.1.2 High
velocity, low amplitude (HVLA) manipulation is performed by taking a joint to
its end range of motion and moving the articulation into the zone of accessory
joint movement, well within the limits of anatomical integrity. Indications for manipulation include joint
pain, decreased joint motion, and joint adhesions. Contraindications to HVLA manipulation
include joint instability, fractures, severe osteoporosis, infection, metastatic
cancer, active inflammatory arthritides, aortic aneurysm, and signs of
progressive neurologic deficits.
6.15.7.1.3 Time
to produce effect for all types of manipulative treatment: 1 to 6 treatments.
6.15.7.1.4 Frequency: Up to 3
times per week for the first 4 weeks as indicated by the severity of
involvement and the desired effect, then up to 2 treatments per week for the
next 4 weeks. For further treatments, twice per week or less to maintain
function.
6.15.7.1.5 Maximum
duration: 30 visits. Extended durations of care beyond what is
considered “maximum” may be necessary in cases of re-injury, interrupted
continuity of care, exacerbation of symptoms, and in those patients with
comorbidities. Refer to the
Chronic Pain Guidelines for care beyond 6 months.
6.15.7.1.6 The
combination of 97140 plus either CMT or OMT code is equal to one visit when
performed on the same day. Any
combination of manual therapeutic intervention exceeding 30 visits (not units)
needs to go to UR. A provider may perform manual therapy and
manipulation on the same day. However,
the provider must include a description of the service and a rationale for why
both services are needed to the same body region on the same day.
6.15.7.2
Mobilization (Joint) /Manipulation
6.15.7.2.1 Mobilization
is passive movement involving oscillatory motions to the involved joints. The
passive mobility is performed in a graded manner (I, II, III, IV, or V), which depicts the speed of the
maneuver. It may include skilled manual
joint tissue stretching. Indications
include the need to improve joint play, improve intracapsular arthrokinematics,
or reduce pain associated with tissue impingement.
6.15.7.2.2 Time
to produce effect: 4 to 6 treatments
6.15.7.2.3 Frequency: 2 to 3 times per week
6.15.7.2.4 Maximum
duration: 30 visits (CPT codes 97124 and 97140 can not exceed 30 visits
in combination).
6.15.7.3 Mobilization (Soft Tissue)
6.15.7.3.1 Mobilization
of soft tissue is the skilled application of manual techniques designed to
normalize movement patterns through the reduction of soft tissue pain and
restrictions. Indications include muscle
spasm around a joint, trigger points, adhesions, and neural compression.
6.15.7.3.2 Nerve
Gliding: consist of a series
of flexion and extension movements of the toes, foot, knee, and hip that produce tension
and longitudinal movement along the length of the sciatic, femoral, obturator, and other nerves of the lower extremity. These
exercises are based on the principle that the tissues of the peripheral nervous
system are designed for movement, and that tension and glide (excursion) of
nerves may have an effect on neurophysiology through alterations in vascular
and axoplasmic flow. Nerve gliding performed on a patient by the clinician should
be reinforced by patient performance of
similar techniques as part of a home exercise program at least twice per day.
6.15.7.3.3 Time to produce
effect: 4 to 6 treatments
6.15.7.3.4 Frequency: 2 to 3 times per week
6.15.7.3.5 Maximum duration: 30 visits (CPT
codes 97124 and 97140 can not exceed 30
visits in
combination).
6.15.8 Massage: Manual
or Mechanical - Massage is manipulation of soft tissue with broad ranging
relaxation and circulatory benefits.
This may include stimulation of acupuncture points and acupuncture
channels (acupressure), application of suction cups and techniques that include
pressing, lifting, rubbing, pinching of soft tissues by or with the
practitioner’s hands. Indications
include edema, muscle spasm, adhesions, the need to improve peripheral
circulation and range of motion, or to increase muscle relaxation and
flexibility prior to exercise.
6.15.8.1 Time to produce effect: Immediate.
6.15.8.2 Frequency: 1 to 3 times per week
6.15.8.3 Maximum duration: 12 visits (CPT
codes 97124 and 97140 can not exceed 30 visits in combination).
6.15.9 Paraffin Bath
is a superficial heating modality that uses melted paraffin (candle
wax) to treat irregular surfaces such as the foot or ankle. Indications include the need to enhance
collagen extensibility before stretching, reduce muscle guarding, or reduce
inflammatory response.
6.15.9.1 Time to Produce Effect: 1 to 4 treatments.
6.15.9.2 Frequency: 1 to 5 times per week.
6.15.9.3 Maximum Duration: 20 visits.
If beneficial, provide with home unit or purchase if effective.
6.15.10 Superficial Heat and Cold Therapy: thermal agents applied in various manners that
lower or raise the body tissue temperature for the reduction of pain,
inflammation, and/or effusion resulting from injury or induced by
exercise. Includes application of heat
just above the surface of the skin at acupuncture points. Indications include acute pain, edema and
hemorrhage, need to increase pain threshold, reduce muscle spasm and promote
stretching/flexibility. Cold and heat
packs can be used at home as an extension of therapy in the clinic setting.
6.15.10.1 Time to produce effect: Immediate
6.15.10.2 Frequency: 2 to 5 times per week (clinic). Home
treatment as needed.
6.15.10.3 Maximum duration:
12 visits with maximum visits of 1 per day. If symptoms persist, consideration should be given to further
diagnostic studies or other treatment options.
6.15.11 Short-wave Diathermy is an accepted treatment which
involves the use of equipment that exposes soft tissue to a magnetic or electrical
field. Indications include enhanced collagen extensibility before stretching,
reduced muscle guarding, reduced inflammatory response, and enhanced
re-absorption of hemorrhage/hematoma or edema. It is an accepted modality as an
adjunct to acupuncture or situation where other forms of contact superficial
heat are contraindicated.
6.15.11.1 Time to produce effect: 2-4 treatments
6.15.11.2 Frequency: 2-3 times per week
6.15.11.3 Maximum Duration: 12 visits.
6.15.12 Therapeutic Taping/Strapping: Fabrication
and application of strapping or taping (e.g. use of elastic wraps, heavy
cloths, or adhesive tape) are used to enhance performance of tasks for
movements, support weak or ineffective joints or muscles, reduce or correct
joint limitations or deformities, and/or protect body parts from injury. Splints and strapping are also used in
conjunction with therapeutic exercise, functional training and other
interventions, and should be selected in the context of the patient’s
need. Examples include, but are not
limited to, strains and sprains of joints, patello femoral syndrome, and post
surgical rehabilitation.
6.15.12.1 Time to produce effect: 3-5 treatments
6.15.12.2 Frequency: Every 1-5 days
6.15.12.3 Maximum Duration: 15 visits per involved area
6.15.13 Transcutaneous Electrical Nerve
Stimulation (TENS) is a generally accepted treatment. TENS should
include at least one instructional session for proper application and use. Indications include muscle spasm, atrophy,
and decreased circulation and pain control.
Minimal TENS unit parameters should include pulse rate, pulse width and
amplitude modulation. Consistent,
measurable functional improvement must be documented prior to the purchase of a
home unit.
6.15.13.1 Time
to Produce Effect: Immediate.
6.15.13.2 Frequency: Variable.
6.15.13.3 Maximum
Duration: 3 sessions. If beneficial, provide with home unit or
purchase if effective.
6.15.14 Ultrasound is
an accepted treatment which includes
ultrasound with electrical stimulation and Phonophoresis. Ultrasound uses sonic generators
to deliver acoustic energy for therapeutic thermal and/or non-thermal soft
tissue effects. Indications include scar
tissue, adhesions, collagen fiber and muscle spasm, and the need to extend
muscle tissue or accelerate the soft tissue healing.
6.15.14.1 Ultrasound with electrical
stimulation is concurrent delivery of electrical energy that involves a
dispersive electrode placement.
Indications include muscle spasm, scar tissue, pain modulation, and muscle
facilitation.
6.15.14.2 Phonophoresis is the transfer
of medication to the target tissue to control inflammation and pain through the
use of sonic generators. These topical
medications include, but are not limited to, steroidal anti-inflammatory and
anesthetics.
6.15.14.3 Time to produce effect: 4 to 8
treatments
6.15.14.4 Frequency: 2-3 times per week
6.15.14.5 Maximum duration: 18 visits
6.15.15 Vasopneumatic Devices are mechanical
compressive devices used in both inpatient and outpatient settings to reduce
various types of edema. Indications
include pitting edema, lymphedema and venostasis. Maximum compression should not exceed minimal
diastolic blood pressure. Use of a unit
at home should be considered if expected treatment is greater than two weeks.
6.15.15.1 Time
to Produce Effect: 1 to 3 treatments.
6.15.15.2 Frequency: 3 to 5 times per week..
6.15.15.3 Maximum
Duration: 24 visits
6.15.16 Whirlpool is conductive exposure to water at temperatures that
best elicits the desired effect (cold vs. heat). It generally includes massage by water
propelled by a turbine or Jacuzzi jet system and has the same thermal effects
as hot packs if higher than tissue temperature.
It has the same thermal effects as cold application if comparable temperature
water used. Indications include the need
for analgesia, relaxing muscle spasm, reducing joint stiffness, enhancing
mechanical debridement and facilitating and preparing for exercise.
6.15.16.1 Time
to Produce Effect: 2 to 4 treatments.
6.15.16.2 Frequency: 3 to 5 times per week.
6.15.16.3 Maximum
Duration: 24 visits
6.16 VOCATIONAL REHABILITATION is a generally
accepted intervention. Initiation of
vocational rehabilitation requires adequate evaluation of patients for
quantification of highest functional level and motivation. Vocational rehabilitation may be as simple as
returning to the original job or as complicated as being retrained for a new
occupation. The effectiveness of
vocational rehabilitation may be enhanced when performed in combination with
work hardening or work conditioning.
|
All operative
interventions must be based upon positive correlation of clinical findings,
clinical course and diagnostic tests. A
comprehensive assimilation of these factors must lead to a specific diagnosis
with positive identification of pathologic condition(s). It is imperative to rule out non-physiologic
modifiers of pain presentation or non-operative conditions mimicking
radiculopathy or instability (e.g., peripheral neuropathy, piriformis syndrome,
myofascial pain, complex regional pain syndrome or sympathetically mediated
pain syndromes, sacroiliac dysfunction, psychological conditions, etc.) prior
to consideration of elective surgical intervention.
In addition,
operative treatment is indicated when the natural history of surgically treated
lesions is better than the natural history for non-operatively treated
lesions. All patients being considered
for surgical intervention should first undergo a comprehensive
neuromusculoskeletal examination to identify mechanical pain generators that
may respond to non-surgical techniques or may be refractory to surgical
intervention.
Structured
rehabilitation interventions are necessary for all of the following procedures
except in some cases of hardware removal.
Return-to-work
restrictions should be specific according to the recommendation in the Section 6.0,
Therapeutic Procedures, Non-operative.
7.1 ANKLE AND SUBTALAR FUSION
7.1.1 Description/Definition:
Surgical fusion of the ankle or subtalar joint.
7.1.2 Occupational Relationship:
Usually post-traumatic arthritis or residual deformity.
7.1.3 Specific Physical Exam Findings:
Painful, limited range of motion of the joint(s). Possible fixed deformity.
7.1.4 Diagnostic Testing Procedures:
Radiographs. Diagnostic
injections, MRI, CT scan, and/or bone scan.
7.1.5 Surgical Indications/Considerations: All reasonable conservative
measures have been exhausted and other reasonable surgical options have been
seriously considered or implemented.
Patient has disabling pain or deformity. Fusion is the procedure of
choice for individuals with osteoarthritis who plan to return to physically
demanding activities.
7.1.5.1 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals, and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
7.1.5.2 Because smokers have a higher risk of
delayed bone healing and post-operative costs, it is recommended that insurers
cover a smoking cessation program peri-operatively. Physicians may monitor smoking cessation with
laboratory tests such as cotinine levels for long-term cessation.
7.1.6 Operative Procedures: Open reduction internal fixation (ORIF) with
possible bone grafting. External
fixation may be used in some cases.
7.1.7 Post-operative Treatment:
7.1.7.1 An individualized rehabilitation program
based upon communication between the surgeon/physician and the therapy provider and using therapies as
outlined in Section 6.0, Therapeutic Procedures, Non-operative. In all cases, communication between the
physician and therapist is important to the timing of weight-bearing, and
exercise progressions.
7.1.7.2 When boney union is achieved, treatment
usually includes active therapy with or without passive therapy, including gait
training and ADLs.
7.1.7.3 Rocker bottom soles or shoe lifts may be
required. A cast is usually in place for 6 to 8 weeks followed by graduated
weight-bearing. Modified duty may last up to 4 to 6 months.
7.1.7.4 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
7.2 KNEE FUSION
7.2.1 Description/Definition: Surgical fusion of femur to the tibia at
the knee joint.
7.2.2 Occupational Relationship: Usually from post-traumatic arthritis or
deformity.
7.2.3 Specific Physical Exam Findings: Stiff, painful, sometime deformed
limb at the knee joint.
7.2.4 Diagnostic Testing Procedures: Radiographs. MRI, CT, diagnostic
injections or bone scan.
7.2.5 Surgical Indications/Considerations: All reasonable conservative
measures have been exhausted and other reasonable surgical options have been
seriously considered or implemented, e.g. failure of arthroplasty. Fusion is a consideration particularly in the
young patient who desires a lifestyle that would subject the knee to high
mechanical stresses. The patient should
understand that the leg will be shortened and there may be difficulty with
sitting in confined spaces, and climbing stairs. Although there is generally a painless knee,
up to 50% of cases may have complications.
7.2.5.1 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
7.2.5.2 Because smokers have a higher risk of
delayed bone healing and post-operative costs, it is recommended that insurers
cover a smoking cessation program peri-operatively. Physicians may monitor smoking cessation with
laboratory tests such as cotinine levels for long-term cessation.
7.2.6 Operative Procedures: Open
reduction internal fixation (ORIF) with possible bone grafting. External fixation or intramedullary rodding
may also be used.
7.2.7 Post-operative Treatment:
7.2.7.1 An individualized rehabilitation program
based upon communication between the surgeon/physician and the therapy provider and using therapies as
outlined in Section 6.0, Therapeutic Procedures, Non-operative. In all cases, communication between the
physician and therapist is important to the timing of weight-bearing, and
exercise progressions.
7.2.7.2 When boney union is achieved, treatment
usually includes active therapy with or without passive therapy, including gait
training and ADLs. Non weight-bearing or
limited weight-bearing and modified duty may last up to 4 and 6 months.
7.2.7.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
7.3 ANKLE ARTHROPLASTY
7.3.1 Description/Definition: Prosthetic replacement of the
articulating surfaces of the ankle joint.
7.3.2 Occupational
Relationship: Usually
from post-traumatic arthritis.
7.3.3 Specific
Physical Exam Findings: Stiff,
painful ankle. Limited range-of-motion
of the ankle joint.
7.3.4 Diagnostic
Testing Procedures: Radiographs,
MRI, diagnostic injections, CT scan, bone scan.
7.3.5 Surgical
Indications/Considerations: When pain interferes with ADLs, and all
reasonable conservative measures have been exhausted and other reasonable
surgical options have been considered or implemented. A very limited population of patients is
appropriate for ankle arthroplasty.
7.3.5.1 Requirements include:
7.3.5.1.1 Good bone quality;
7.3.5.1.2 BMI less than 35;
7.3.5.1.3 Non-smoker
currently;
7.3.5.1.4 Patient is 60 or older;
7.3.5.1.5 No lower extremity
neuropathy;
7.3.5.1.6 Patient does not pursue
physically demanding work or recreational activities.
7.3.5.2 The following issues should be addressed
when determining appropriateness for surgery: ankle laxity, bone alignment,
surrounding soft tissue quality, vascular status, presence of avascular
necrosis, history of open fracture or infection, motor dysfunction, and
treatment of significant knee or hip pathology.
7.3.5.3 Ankle implants are less
successful than similar procedures in the knee or hip. There are no good studies comparing
arthrodesis and ankle replacement.
Patients with ankle fusions generally have good return to function and
fewer complications than those with joint replacements. Re-operation rates may be higher in ankle
arthroplasty than in ankle arthrodesis.
Long-term performance beyond ten years for current devices is still
unclear. Salvage procedures for ankle replacement include revision with stemmed
implant or allograft fusion. Given these factors, an ankle arthroplasty
requires prior authorization and a second opinion by a surgeon specializing in
lower extremity surgery.
7.3.5.4 Contraindications
include severe osteoporosis, significant general disability due to other
medical conditions, psychiatric issues.
7.3.5.4.1 In cases where surgery is
contraindicated due to obesity, it may be appropriate to recommend a weight
loss program if the patient is unsuccessful losing weight on their own.
Coverage for weight loss would continue only for motivated patients who have
demonstrated continual progress with weight loss.
7.3.5.5 Prior to surgery, patients may be
assessed for any associated mental health or low back pain issues that may
affect rehabilitation.
7.3.5.6 Prior to surgical intervention, the
patient and treating physician should identify functional operative goals and
the likelihood of achieving improved ability to perform activities of daily
living or work activities and the patient should agree to comply with the pre-
and post-operative treatment plan including home exercise. The provider should
be especially careful to make sure the patient understands the amount of
post-operative therapy required and the length of partial- and full-disability
expected post-operatively.
7.3.5.7 Because smokers have a higher risk of
delayed bone healing and post-operative costs, it is recommended that insurers
cover a smoking cessation program peri-operatively. Physicians may monitor smoking cessation with
laboratory tests such as cotinine levels for long-term cessation.
7.3.6 Operative
Procedures: Prosthetic replacement of the articular surfaces of the
ankle; DVT prophylaxis is not always required but should be considered for
patients who have any risk factors for thrombosis.
7.3.6.1 Complications
– include pulmonary embolism, infection, bony lysis, polyethylene wear,
tibial loosening, instability, malalignment, stiffness, nerve-vessel injury,
and peri-prosthetic fracture.
7.3.7 Post-operative
Treatment:
7.3.7.1 An individualized rehabilitation program
based upon communication between the surgeon/physician and the therapy provider while using therapies as
outlined in Section 6.0, Therapeutic Procedures, Non-operative. In all cases, communication between the
physician and therapist is important to the timing of weight-bearing, and
exercise progressions.
7.3.7.2 NSAIDs may be used for pain management after
joint replacement. They have also been used to reduce heterotopic ossification
after ankle arthroplasty. NSAIDs do reduce the radiographically documented
heterotopic ossification in this setting, but there is some evidence (in
literature on hip arthroplasty) that they do not improve functional outcomes
and they may increase the risk of bleeding events in the post-operative period.
Their
routine use for prevention of heterotopic bone formation is not recommended.
7.3.7.3 Treatment may include the following:
bracing, active therapy with or without passive therapy, gait training, and
ADLs. Rehabilitation post-operatively
may need to be specifically focused based on the following problems:
contracture, gastrocnemius muscle weakness, and foot and ankle malalignment.
Thus, therapies may include braces, shoe lifts, orthoses, and electrical
stimulation accompanied by focused therapy.
7.3.7.4 In some cases aquatic therapy may be
used. Refer to Section 6.0, Therapeutic Procedures, Non-operative 14, b,
Aquatic Therapy. Pool exercises may be done initially under therapist's or surgeon's
direction then progressed to an independent pool program.
7.3.7.5 Prior to revision surgery
there should be an evaluation to rule out infection.
7.3.7.6 Return to work and restrictions after
surgery may be made by a treating physician in consultation with the surgeon or
by the surgeon. Patient should be able to return to sedentary work within 4 to
6 weeks. Some patients may have
permanent restrictions based on their job duties.
7.3.7.7 Patients are usually seen annually after
initial recovery to check plain x-rays for signs of loosening.
7.4 KNEE ARTHROPLASTY
7.4.1 Description/Definition: Prosthetic replacement of the articulating
surfaces of the knee joint.
7.4.2 Occupational Relationship: Usually from post-traumatic
osteoarthritis.
7.4.3 Specific Physical Exam Findings: Stiff, painful knee, and possible
effusion.
7.4.4 Diagnostic Testing Procedures: Radiographs.
7.4.5 Surgical Indications/Considerations: Severe osteoarthritis and all
reasonable conservative measures have been exhausted and other reasonable
surgical options have been considered or implemented. Significant changes such as advanced joint line narrowing
are expected. Refer to Aggravated
Osteoarthritis in Section 5.0
7.4.5.1 Younger patients, less than 50 years of
age, may be considered for unicompartmental replacement if there is little or
no arthritis in the lateral compartment, there is no inflammatory disease
and/or deformity and BMI is less than 35. They may be considered for lateral
unicompartmental disease when the patient is not a candidate for osteotomy.
Outcome is better for patients with social support.
7.4.5.2 Contraindications
- severe osteoporosis, significant general disability due to other medical
conditions, psychiatric issues.
7.4.5.2.1 In cases where surgery is
contraindicated due to obesity, it may be appropriate to recommend a weight
loss program if the patient is unsuccessful losing weight on their own.
Coverage for weight loss would continue only for motivated patients who have
demonstrated continual progress with weight loss.
7.4.5.3 Prior to surgery, patients may be
assessed for any associated mental health or low back pain issues that may
affect rehabilitation.
7.4.5.4 Prior
to surgical intervention, the patient and treating physician should identify
functional activities and the patient
should agree to comply with the pre- and post-operative treatment plan
including home exercise. The
provider should be especially careful to make sure the patient understands the
amount of post-operative therapy required and the length of partial- and
full-disability expected post-operatively.
7.4.5.5 Because smokers have a higher risk of
delayed bone healing and post-operative costs, it is recommended that insurers
cover a smoking cessation program peri-operatively. Physicians may
monitor smoking cessation with laboratory tests such as cotinine levels for
long-term cessation.
7.4.6 Operative Procedures:
Prosthetic replacement of the articular surfaces of the knee; total or uni-compartmental with DVT
prophylaxis. May include patellar
resurfacing and computer assistance.
7.4.6.1 There is currently conflicting evidence
on the effectiveness of patellar resurfacing. Isolated patellofemoral
resurfacing is performed on patients under 60 only after diagnostic arthroscopy
does not reveal any arthritic changes in other compartments. The diagnostic arthroscopy is generally
performed at the same time as the resurfacing. Resurfacing may accompany a
total knee replacement at the discretion of the surgeon.
7.4.6.2 Computer guided implants are more likely
to be correctly aligned. The overall
long-term functional result using computer guidance is unclear. Decisions to use computer assisted methods
depend on surgeon preference and age of the patient as it is more likely to
have an impact on younger patients with longer expected use and wear of the
implant. Alignment is only one of many factors that may affect the implant
longevity.
7.4.6.3 Complications
occur in around 3% and include pulmonary embolism; infection, bony lysis,
polyethylene wear, tibial loosening, instability, malalignment, stiffness,
patellar tracking abnormality, nerve-vessel injury, and peri-prosthetic
fracture.
7.4.7 Post-operative Treatment:
7.4.7.1 Anti coagulant therapy to prevent deep
vein thrombosis. Refer to Section 6.0,
Therapeutic Procedures, Non-operative.
7.4.7.2 NSAIDs may be used for pain management after
joint replacement. They have also been used to reduce heterotopic ossification
after knee arthroplasty. NSAIDs do reduce the radiographically documented
heterotopic ossification in this setting, but there is some evidence (in
literature on total hip arthroplasty) that they do not improve functional
outcomes and they may increase the risk of bleeding events in the
post-operative period. Their routine use for prevention of
heterotopic bone formation is not recommended.
7.4.7.3 An individualized rehabilitation program
based upon communication between the surgeon/physician and the therapy provider and using therapies as
outlined in Section 6.0, Therapeutic Procedures, Non-operative. In all cases, communication between the
physician and therapist is important to the timing of weight-bearing, and
exercise progressions.
7.4.7.4 Treatment may include the following:
bracing and active therapy with or without passive therapy. Rehabilitation post-operatively may need to
be specifically focused based on the following problems: knee flexion
contracture, quadriceps muscle weakness, knee flexion deficit, and foot, and
ankle malalignment. Thus, therapies may include, knee braces, shoe lifts,
orthoses, and electrical stimulation, accompanied by focused active
therapy.
7.4.7.5 In some cases aquatic therapy may be
used. Refer to Section 6.0,. Therapeutic Procedures, Non-operative, Aquatic
Therapy. Pool exercises may be done
initially under a therapy provider’s or surgeon's/physician’s direction then
progressed to an independent pool program.
7.4.7.6 Continuous passive motion is frequently
prescribed. The length of time it is used will depend on the patient and their
ability to return to progressive exercise.
7.4.7.7 Consider need for manipulation under
anesthesia if there is less than 90 degrees of knee flexion after 6 weeks.
7.4.7.8 Prior to revision surgery
there should be an evaluation to rule out infection.
7.4.7.9 Return to work and restrictions after surgery
may be made by an attending physician in consultation with the surgeon or by
the surgeon. Patient should be able to return to sedentary work within 4 to 6
weeks. Some patients may have permanent
restrictions based on their job duties.
7.4.7.10 Patients are usually seen annually after
initial recovery to check plain x-rays for signs of loosening.
7.5 HIP ARTHROPLASTY
7.5.1 Description/Definition: Prosthetic replacement of the articulating
surfaces of the hip joint. In some cases, hip resurfacing may be performed.
7.5.2 Occupational Relationship: Usually from post-traumatic arthritis,
hip dislocations and femur or acetabular fractures. Patients with intracapsular femoral fractures
have a risk of developing avascular necrosis of the femoral head requiring
treatment months to years after the initial injury.
7.5.3 Specific Physical Exam Findings: Stiff, painful hip.
7.5.4 Diagnostic Testing Procedures: Standing pelvic radiographs
demonstrating joint space narrowing to 2 mm or less, osteophytes or sclerosis
at the joint. MRI may be ordered to rule
out other more serious disease.
7.5.5 Surgical Indications/Considerations: Severe osteoarthritis and all reasonable
conservative measures have been exhausted and other reasonable surgical options
have been considered or implemented. Refer to Aggravated Osteoarthritis in
Section 5.0
7.5.5.1 Possible contraindications - inadequate bone density, prior hip
surgery, and obesity.
7.5.5.1.1 In cases where surgery is
contraindicated due to obesity, it may be appropriate to recommend a weight
loss program if the patient is unsuccessful losing weight on their own.
Coverage for weight loss would continue only for motivated patients who have
demonstrated continual progress with weight loss.
7.5.5.2 Prior to surgery, patients may be
assessed for any associated mental health or low back pain issues that may
affect rehabilitation.
7.5.5.3 For patients undergoing total hip
arthroplasty, there is some evidence that a pre-operative exercise conditioning
program, including aquatic and land-based exercise, results in quicker
discharge to home than pre-operative education alone without an exercise
program.
7.5.5.4 Aseptic loosening of the joint requiring
revision surgery occurs in some patients.
Prior to revision the joint should be checked to rule out possible
infection which may require a bone scan as well as laboratory procedures,
including a radiologically directed joint aspiration.
7.5.5.5 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that carriers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as
cotinine levels for long-term cessation.
7.5.6 Operative Procedures: Prosthetic replacement of the articular
surfaces of the hip, ceramic or metal prosthesis, with DVT prophylaxis. Ceramic prosthesis is more expensive;
however, it is expected to have greater longevity and may be appropriate in
some younger patients. Hip resurfacing,
metal on metal, is an option for younger or active patients likely to out-live
traditional total hip replacements.
7.5.6.1 Complications
include leg length inequality, deep venous thrombosis with possible
pulmonary embolus, hip dislocation, possible renal effects, need for
transfusions, future infection, need for revisions, fracture at implant site.
7.5.6.2 The long-term benefit for computer
assisted hip replacements is unknown. It
may be useful in younger patients. Prior
authorization is required.
7.5.6.3 Robotic assisted surgery is considered
experimental and not recommended due to technical difficulties.
7.5.7 Post-operative Treatment:
7.5.7.1 Anti coagulant therapy is
used to prevent deep vein thrombosis.
Refer to Section 6.0, Therapeutic Procedures, Non-operative.
7.5.7.2 NSAIDs may be used for pain management after
joint replacement. They have also been used to reduce heterotopic ossification
after hip arthroplasty. NSAIDs do reduce the radiographically documented
heterotopic ossification in this setting, but there is some evidence that they
do not improve functional outcomes and they may increase the risk of bleeding
events in the post-operative period. Their routine use for prevention of
heterotopic bone formation is not recommended.
7.5.7.3 An individualized rehabilitation program
based upon communication between the surgeon/physician and the therapy provider
and using the therapies as outlined in
Section 6.0, Therapeutic Procedures Non-operative. In all cases, communication between the
physician and therapist is important to the timing of weight-bearing and
exercise progressions.
7.5.7.4 Treatment usually includes active
therapy with or without passive therapy with emphasis on gait training with
appropriate assistive devices. Patients
with accelerated return to therapy appear to do better. Therapy should include
training on the use of adaptive equipment and home and work site evaluation
when appropriate.
7.5.7.4.1 There is good evidence for the use of
aquatic therapy. Refer to Section 6.0, Therapeutic
Procedures, Non-operative. Pool
exercises may be done initially under a therapy provider’s or surgeon's/physician’s
direction then progressed to an independent pool program.
7.5.7.4.2 There is some evidence that, for
patients older than 60, early multidisciplinary therapy may shorten hospital
stay and improve activity level for those receiving hip replacement. Therefore, this may be used for selected
patients.
7.5.7.5 Return to activities at 4 to 6 weeks
with appropriate restrictions by the surgeon. Initially range of motion is
usually restricted. Return to activity
after full recovery depends on the surgical approach. Patients can usually lift, but jogging and other
high impact activities are avoided.
7.5.7.6 Helical CT or MRI with artifact
minimization may be used to investigate prosthetic complications. The need for implant revision is determined
by age, size of osteolytic lesion, type of lesion and functional status.
Revision surgery may be performed by an orthopedic surgeon in cases with
chronic pain and stiffness or difficulty with activities of daily living. Prior
authorization is required and a second opinion by a surgeon with special
expertise in hip/knee replacement surgery should usually be performed.
7.5.7.7 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
7.5.7.8 Patients are usually seen annually after
the initial recovery to check plain x-rays for signs of loosening.
7.6 AMPUTATION
7.6.1 Description/Definition: Surgical removal of a portion of the lower
extremity.
7.6.2 Occupational Relationship: Usually secondary to post-traumatic
bone, soft tissue, vascular or neurologic compromise of part of the extremity.
7.6.3 Specific Physical Exam Findings: Non-useful or non-viable portion
of the lower extremity.
7.6.4 Diagnostic
Testing Procedures: Radiographs, vascular studies, MRI, bone scan.
7.6.5 Surgical Indications/Considerations: Non-useful or non-viable
portion of the extremity.
7.6.5.1 Smoking may affect soft tissue healing
through tissue hypoxia. Patients should be strongly encouraged to stop smoking
and be provided with appropriate counseling by the physician.
7.6.6 Operative Procedures:
Amputation.
7.6.7 Post-operative Treatment:
7.6.7.1 An individualized rehabilitation program
based upon communication between the surgeon/physician and the therapy provider
and using therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative.
7.6.7.2 Rigid removable dressings
are used initially.
7.6.7.3 Therapies usually include active therapy
with or without passive therapy for prosthetic fitting, construction and
training, protected weight-bearing, training on the use of adaptive equipment,
and home and jobsite evaluation. Temporary prosthetics are used initially with
a final prosthesis fitted by the second year.
Multiple fittings and trials may be necessary to assure the best
functional result.
7.6.7.4 For prosthesis with special adaptive
devices, e.g. computerized prosthesis; prior authorization and a second opinion
from a physician knowledgeable in prosthetic rehabilitation and who has a clear
description of the patients expected job duties and daily living activities are
required.
7.6.7.5 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
7.7 MANIPULATION UNDER ANESTHESIA
7.7.1 Description/Definition: Passive range of motion of a joint under
anesthesia.
7.7.2 Occupational Relationship: Typically from joint stiffness that
usually results from a traumatic injury, compensation related surgery, or other
treatment.
7.7.3 Specific Physical Exam Findings: Joint stiffness in both active and
passive modes.
7.7.4 Diagnostic Testing Procedures: Radiographs. CT, MRI, diagnostic
injections.
7.7.5 Surgical Indications/Considerations: Consider if routine
therapeutic modalities, including therapy and/or dynamic bracing, do not
restore the degree of motion that should be expected after a reasonable period
of time, usually at least 12 weeks.
7.7.6 Operative Treatment: Not applicable.
7.7.7 Post-operative Treatment:
7.7.7.1 An individualized rehabilitation program
based upon communication between the physician/surgeon and the therapy provider
and using therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative. Therapy includes a
temporary increase in frequency of both active and passive therapy to maintain
the range of motion gains from surgery.
7.7.7.2 Continuous passive motion is
frequently used post-operatively.
7.7.7.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
7.8 OSTEOTOMY
7.8.1 Description/Definition: A reconstructive procedure involving the
surgical cutting of bone for realignment.
It is useful for patients that would benefit from realignment in lieu of
total joint replacement.
7.8.2 Occupational Relationship: Usually, post-traumatic arthritis or
deformity.
7.8.3 Specific Physical Exam Findings: Painful decreased range of motion
and/or deformity.
7.8.4 Diagnostic Testing Procedures: Radiographs, MRI scan, CT scan.
7.8.5 Surgical Indications/Considerations: Failure of non-surgical treatment when
avoidance of total joint arthroplasty is desirable. For the knee, joint
femoral osteotomy may be desirable for young or middle age patients with varus
alignment and medial arthritis or valgus alignment and lateral compartment
arthritis. High tibial osteotomy is also used for medial compartment arthritis.
Multi-compartmental degeneration is a contraindication. Patients should have a
range of motion of at least 90 degrees of knee flexion. For the ankle supra malleolar osteotomy may
be appropriate. High body mass is a relative contraindication.
7.8.5.1 Because smokers have a higher risk of
non-union and post-operative costs, it is recommended that carriers cover a
smoking cessation program peri-operatively.
Physicians may monitor smoking cessation with laboratory tests such as cotinine
levels for long-term cessation.
7.8.6 Operative Procedures: Peri-articular opening or closing wedge of
bone, usually with grafting and internal or external fixation.
7.8.6.1 Complications
- new fractures, lateral peroneal
nerve palsy, infection, delayed unions, compartment syndrome, or pulmonary
embolism.
7.8.7 Post-operative Treatment:
7.8.7.1 An individualized rehabilitation program
based upon communication between the physician/surgeon and the therapy provider
and using therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative. In all cases, communication between the physician and therapist
is important to the timing of weight-bearing, and exercise progressions.
7.8.7.2 Weight-bearing and range-of-motion
exercises depend on the type of procedure performed. Partial or full weight-bearing restrictions
can range from 6 weeks partial weight-bearing, to 3 months full
weight-bearing. It is usually 6 months
before return to sports or other rigorous physical activity.
7.8.7.3 If
femoral intertrochanteric osteotomy has been performed, there is some evidence
that electrical bone growth stimulation may improve bone density. Refer to
Section 6.0, Therapeutic Procedures, Non-operative, Bone Growth Stimulators for
description.
7.8.7.4 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
7.9 HARDWARE
REMOVAL Hardware removal frequently occurs after initial MMI.
Physicians should document the possible need for hardware removal and include
this as treatment in their final report on the WC 164 form.
7.9.1 Description/Definition:
Surgical removal of internal or external fixation device, commonly related to
fracture repairs.
7.9.2 Occupational
Relationship: Usually following healing of a post-traumatic injury
that required fixation or reconstruction using instrumentation.
7.9.3 Specific
Physical Exam Findings: Local pain to palpation, swelling, erythema.
7.9.4 Diagnostic
Testing Procedures: Radiographs, tomography, CT scan, MRI.
7.9.5 Surgical
Indications/Considerations: Persistent local pain, irritation around
hardware.
7.9.6 Operative
Procedures: Removal of hardware may be accompanied by scar
release/resection, and/or manipulation. Some instrumentation may be removed in the
course of standard treatment without symptoms of local irritation.
7.9.7 Post-operative Treatment:
7.9.7.1 An individualized rehabilitation program
based upon communication between the physician/surgeon and the therapy provider
and using therapies as outlined in Section 6.0, Therapeutic Procedures,
Non-operative.
7.9.7.2 Treatment may include therapy with or
without passive therapy for progressive weight-bearing, range of motion.
7.9.7.3 Return to work and restrictions after
surgery may be made by an attending physician in consultation with the surgeon
or by the surgeon.
7.10 RELEASE OF CONTRACTURE
7.10.1 Description/Definition: Surgical incision or lengthening of
contracted tendon or peri-articular soft tissue.
7.10.2 Occupational Relationship: Usually following a post-traumatic
complication.
7.10.3 Specific Physical Exam Findings: Shortened tendon or stiff joint.
7.10.4 Diagnostic Testing Procedures: Radiographs, CT scan, MRI scan.
7.10.5 Surgical Indications/Considerations: Persistent shortening or
stiffness associated with pain and/or altered function.
7.10.5.1 Smoking may affect soft tissue
healing through tissue hypoxia. Patients should be strongly encouraged to stop
smoking and be provided with appropriate counseling by the physician.
7.10.6 Operative Procedures: Surgical incision or lengthening of involved
soft tissue.
7.10.7 Post-operative Treatment:
7.10.7.1 An individualized
rehabilitation program based upon communication between the physician/surgeon and
the therapy provider and using therapies as outlined in Section 6.0,
Therapeutic Procedures, Non-operative.
7.10.7.2 Treatments may include active
therapy with or without passive therapy for stretching, range of motion
exercises.
7.10.7.3 Return to work and
restrictions after surgery may be made by an attending physician in
consultation with the surgeon or by the surgeon.
7.11 Human Bone Morphogenetic Protein (RhBMP): (RhBMP)
is a member of a family of proteins which are involved in the growth, remodeling,
and regeneration of bone tissue. It has
become available as a recombinant biomaterial with osteo-inductive potential
for application in long bone fracture non-union and other situations in which
the promotion of bone formation is desired.
RhBMP may be used with
intramedullary rod treatment for open tibial fractures an open tibial Type III A and B fracture treated with an intramedullary
rod. There is some evidence that it decreases the need for further procedures
when used within 14 days of the injury. It should not be used in those
with allergies to the preparation, or in females with the possibility of child
bearing, or those without adequate neurovascular status or those less than 18
years old. Ectopic ossification into adjacent muscle has been reported
to restrict motion in periarticular fractures.
Other than for tibial open fractures as described above, it should be
used principally for non-union of fractures that have not healed with
conventional surgical management or peri-prosthetic fractures.
|
|
|